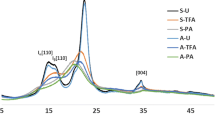
Abstract
Dehydration of wet bacterial cellulose (BC) mainly focuses on the physical characteristic or mechanical strength, but its polymorph crystal structure and thermal properties were rarely discussed. Herein, this study utilizes the drying method of hot-pressed and freeze-dried to the wet BC to elucidate the BC polymorph crystal structure in correlation with the BC crystallinity and thermal properties. The results indicate that dehydration of BC using hot-pressed (BC-HP) method reduced the cellulose Iα allomorph fraction by ± 5.0%, increased the crystallinity by ± 5.0%, but lower in thermal stability compared to freeze-dried (BC-FD) method. Additionally, BC-HP shows an exothermic reaction of melting at lower enthalpy value which is contradict to BC-FD and plant cellulose that presented an endothermic reaction with higher enthalpy values. Thus, investigation on BC polymorph crystal structure and thermal properties may offer an important role to regulate the desired characteristic of the dried BC.






Similar content being viewed by others
References
Revin V, Liyaskina E, Nazarkina M et al (2018) Cost-effective production of bacterial cellulose using acidic food industry by-products. Brazilian J Microbiol 49:151–159. https://doi.org/10.1016/j.bjm.2017.12.012
Mohammadkazemi F, Azin M, Ashori A (2015) Production of bacterial cellulose using different carbon sources and culture media. Carbohydr Polym 117:518–523. https://doi.org/10.1016/j.carbpol.2014.10.008
Shezad O, Khan S, Khan T, Park JK (2010) Physicochemical and mechanical characterization of bacterial cellulose produced with an excellent productivity in static conditions using a simple fed-batch cultivation strategy. Carbohydr Polym 82:173–180. https://doi.org/10.1016/j.carbpol.2010.04.052
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chemie Int Ed 44:3358–3393. https://doi.org/10.1002/anie.200460587
Lin D, Liu Z, Shen R et al (2020) Bacterial cellulose in food industry: current research and future prospects. Int J Biol Macromol 158:1007–1019. https://doi.org/10.1016/j.ijbiomac.2020.04.230
De AI, Fernandes A, Cristina A et al (2020) Bacterial cellulose: from production optimization to new applications. Int J Biol Macromol 164:2598–2611. https://doi.org/10.1016/j.ijbiomac.2020.07.255
Kosseva MR, Zhong S, Li M et al (2020) Biopolymers produced from food wastes: a case study on biosynthesis of bacterial cellulose from fruit juices. Food Ind Wastes. https://doi.org/10.1016/b978-0-12-817121-9.00011-5
Illa MP, Sharma CS, Khandelwal M (2019) Tuning the physiochemical properties of bacterial cellulose: effect of drying conditions. J Mater Sci 54:12024–12035. https://doi.org/10.1007/s10853-019-03737-9
Clasen C, Sultanova B, Wilhelms T et al (2006) Effects of different drying processes on the material properties of bacterial cellulose membranes. Macromol Symp 244:48–58. https://doi.org/10.1002/masy.200651204
Klemm D, Cranston ED, Fischer D et al (2018) Nanocellulose as a natural source for groundbreaking applications in materials science: today’s state. Mater Today 21:720–748. https://doi.org/10.1016/j.mattod.2018.02.001
Andree V, Niopek D, Müller C et al (2021) Influence of drying methods on the physical properties of bacterial nanocellulose. Mater Res Express. https://doi.org/10.1088/2053-1591/abe016
Hashim NA, Zakaria J, Mohamad S, Mohamad SF et al (2021) Effect of different treatment methods on the purification of bacterial cellulose produced from OPF juice by Acetobacter Xylinum. IOP Conf Ser Mater Sci Eng 1092:012058. https://doi.org/10.1088/1757-899x/1092/1/012058
Sharifah Fathiyah SM, Shahril M, Junaidi Z (2021) Oil palm frond juice and coconut water as alternative fermentation substrate for bacterial cellulose production. IOP Conf Ser Mater Sci Eng 1092:012055. https://doi.org/10.1088/1757-899x/1092/1/012055
Nada AMA, Shabaka AA, Yousef MA, Abd-El-Nour KN (1990) Infrared spectroscopic and dielectric studies of swollen cellulose. J Appl Polym Sci 40:731–739. https://doi.org/10.1002/app.1990.070400510
Kataoka Y, Kondo T (1999) Quantitative analysis for the cellulose Iα crystalline phase in developing wood cell walls. Int J Biol Macromol 24:37–41. https://doi.org/10.1016/S0141-8130(98)00065-8
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An Empirical method for estimating the degree of crystallinity of native cellulose using the X-Ray diffractometer. Text Res J 29:786–794. https://doi.org/10.1177/004051755902901003
Park S, Baker JO, Himmel ME et al (2010) Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:1–10. https://doi.org/10.1186/1754-6834-3-10
Bragg WH, Bragg WL (1913) The reflection of X-rays by crystals. Proc R Soc Lond A 88:428–438. https://doi.org/10.1098/rspa.1913.0040
Patterson AL (1939) The scherrer formula for X-ray particle size determination. Phys Rev 56:978–982. https://doi.org/10.1103/PhysRev.56.978
Wada M, Okano T, Sugiyama J (2001) Allomorphs of native crystalline cellulose I evaluated by two equatorial d-spacings. J Wood Sci 47:124–128. https://doi.org/10.1007/BF00780560
Revin VV, Pestov NA, Shchankin MV et al (2019) A study of the physical and mechanical properties of aerogels obtained from bacterial cellulose. Biomacromol 20:1401–1411. https://doi.org/10.1021/acs.biomac.8b01816
O’connor RT, Dupré EF, Mitcham D (1958) Applications of infrared absorption spectroscopy to investigations of cotton and modified cottons: part I—physical and crystalline modifications and oxidation. Text Res J 28:382–392. https://doi.org/10.1177/004051755802800503
Andritsou V, De Melo EM, Tsouko E et al (2018) Synthesis and characterization of bacterial cellulose from citrus-based sustainable resources. ACS Omega 3:10365–10373. https://doi.org/10.1021/acsomega.8b01315
Sugiyama J, Vuong R, Chanzy H (1991) Electron diffraction study on the two crystalline phases occurring in native cellulose from an algal cell wall. Macromolecules 24:4168–4175. https://doi.org/10.1021/ma00014a033
Heiner AP, Sugiyama J, Teleman O (1995) Crystalline cellulose Iα and Iβ studied by molecular dynamics simulation. Carbohydr Res 273:207–223. https://doi.org/10.1016/0008-6215(95)00103-Z
Vazquez A, Foresti ML, Cerrutti P, Galvagno M (2013) Bacterial cellulose from simple and low cost production media by Gluconacetobacter xylinus. J Polym Environ 21:545–554. https://doi.org/10.1007/s10924-012-0541-3
Qiao N, Fan X, Zhang X et al (2019) Soybean oil refinery effluent treatment and its utilization for bacterial cellulose production by Gluconacetobacter xylinus. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2019.105185
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896. https://doi.org/10.1007/s10570-013-0030-4
Chen G, Wu G, Chen L et al (2019) Performance of nanocellulose-producing bacterial strains in static and agitated cultures with different starting pH. Carbohydr Polym 215:280–288. https://doi.org/10.1016/j.carbpol.2019.03.080
Agarwal UP, Ralph SA, Baez C et al (2017) Effect of sample moisture content on XRD-estimated cellulose crystallinity index and crystallite size. Cellulose 24:1971–1984. https://doi.org/10.1007/s10570-017-1259-0
Andersson S, Serimaa R, Paakkari T et al (2003) Crystallinity of wood and the size of cellulose crystallites in Norway spruce (Picea abies). J Wood Sci 49:531–537. https://doi.org/10.1007/s10086-003-0518-x
Tyagi N, Suresh S (2016) Production of cellulose from sugarcane molasses using Gluconacetobacter intermedius SNT-1: optimization & characterization. J Clean Prod 112:71–80. https://doi.org/10.1016/j.jclepro.2015.07.054
Watanabe K, Tabuchi M, Morinaga Y, Yoshinaga F (1998) Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose 5:187–200. https://doi.org/10.1023/A:1009272904582
Chen QJ, Zhou LL, Zou JQ, Gao X (2019) The preparation and characterization of nanocomposite film reinforced by modified cellulose nanocrystals. Int J Biol Macromol 132:1155–1162. https://doi.org/10.1016/j.ijbiomac.2019.04.063
Castro C, Zuluaga R, Putaux JL et al (2011) Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr Polym 84:96–102. https://doi.org/10.1016/j.carbpol.2010.10.072
Wada M, Okano T (2001) Localization of Iα and Iβ phases in algal cellulose revealed by acid treatments. Cellulose 8:183–188. https://doi.org/10.1023/A:1013196220602
Wada M, Okano T, Sugiyama J (1997) Synchrotron-radiated X-ray and neutron diffraction study of native cellulose. Cellulose 4:221–232. https://doi.org/10.1023/A:1018435806488
Dai H, Ou S, Huang Y, Huang H (2018) Utilization of pineapple peel for production of nanocellulose and film application. Cellulose 25:1743–1756. https://doi.org/10.1007/s10570-018-1671-0
Blanco A, Monte MC, Campano C et al (2018) Nanocellulose for industrial use. Elsevier
Shah N, Ul-Islam M, Khattak WA, Park JK (2013) Overview of bacterial cellulose composites: a multipurpose advanced material. Carbohydr Polym 98:1585–1598. https://doi.org/10.1016/j.carbpol.2013.08.018
Acknowledgements
The research was funded by the Ministry of Higher Education under Fundamental Research Grant Scheme No. FRGS/1/2018/TK05/UMP/03/3 (RDU190154). The authors would like to acknowledge Universiti Putra Malaysia (UPM) and Universiti Malaysia Pahang (UMP) for the technical support during the study period. Finally, our gratitude also goes to UMP and MOHE for the provision of study leave and scholarship for the first author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Handling Editor: Stephen Eichhorn.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamad, S., Abdullah, L.C., Jamari, S.S. et al. Influence of drying method on the crystal structure and thermal property of oil palm frond juice-based bacterial cellulose. J Mater Sci 57, 1462–1473 (2022). https://doi.org/10.1007/s10853-021-06685-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06685-5