Abstract
Particle coalescence refers to the dispersed particles in a suspension sticking to each other through the random collisions. This phenomenon is of vital importance for the process control and mechanical property of the metallic materials, such as Iron–Nickel binary alloy. The present work performed a fundamental study of the composition evolution and coalescence behavior of the Ti-oxide particles in the liquid Iron–Nickel binary alloy. The effect of the titanium addition amount on the composition of the inclusion particles is investigated through the Ti deoxidation experiments. The particle features are characterized by using a potentiostatic electrolytic extraction method. It shows that when the amount of the Ti addition arrives at a certain degree, the state of the oxide particles changes from the liquid to the solid. Meanwhile, the formation of the cluster can occur. The coalescence efficiency and attraction forces of the particles are calculated theoretically. It is found that the coalescence degree of the solid TiOx (x = 1.5–1.67) particle is close to that of the Al2O3 particle. The initial sintering behavior of the particles after coalescence–collision is analyzed by measuring the sintered neck radius. The apparent self-diffusion of the TiOx (x = 1.5–1.67) particle is approximately 1.7 times larger than that of the Al2O3 particle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the excellent mechanical and magnetic properties, Fe–Ni (Ni = 10–30%) alloy has an extensive application in different fields, e.g., magnetic and inductive device manufacturing [1,2,3]. Various research issues of Fe–(10–30%)Ni alloy have been performed in previous studies [4,5,6,7,8]. For instance, Li et al. [4] investigated the solidification structure of the undercooled alloy with different Ni addition. Zeng [5] reported the martensitic structure and hardness in the as-quenched Fe–Ni alloy. Nakada [6] directly observed the martensitic reversion from lenticular martensite to austenite in Fe–29%Ni alloy. Sato and Zaefferer [7] reported the formation mechanism of butterfly-type martensite in this ferrous alloy using EBSD-based orientation microscopy. Besides the research focusing on the solidification and microstructure, the alloy preparation process is vital. One of the most important points in the Fe–Ni alloy manufacturing is to control and optimize the coalescence behavior of the undesirable non-metallic particles in the melt [8]. The particle coalescence in one fluid is of interest in many important processes. It normally occurs among the particles or droplets with the size range from nanometer to several tens of microns [9]. Different stirring methods, for instance Ar-gas stirring and inductive stirring, can promote the coalescence–collision frequency of the particles. Meanwhile, the chemical composition of the particles can also affect the coalescence behavior due to the interfacial energy. The specific interest of this work is to understand the composition evolution and coalescence behavior of the titanium oxide particles in the liquid Iron–Nickel binary alloy.
It is well known that the precipitation of the non-metallic particles, referred as ‘inclusion’ from herein, is un-avoided due to the effects of such interstitial element such as oxygen. Many research investigations [10,11,12,13,14,15] have been performed to investigate the behavior of the non-metallic inclusion in the liquid metal. One effective solution of optimizing the inclusion coalescence is to use the complex deoxidation [11,12,13,14,15]. A weaker deoxidizer (e.g., Ti) is firstly added into the melt to form the oxides. Afterward, a stronger deoxidizer (e.g., Al, Mg, Ca) is added to further decrease the oxygen content in the melt [11]. The addition amount of the deoxidizers needs to be carefully evaluated. As a typical example, different oxide phases can be precipitated with different amount of the titanium addition. A large number of works have been reported [16,17,18,19,20,21] to describe the equilibrium reactions in the Fe–Ti–O system. However, a detailed study of the TiOx inclusion behavior with different amount of the titanium addition is not available in the open literature. In this work, different amount of titanium is used in the laboratory-scale deoxidation experiments to identify a precipitation boundary of the different phases. Furthermore, the coalescence behavior of the inclusions is analyzed through both the experimental observation and theoretical consideration. The obtained understanding can be applied in a comprehensive study of the particle behavior in different metallic alloys, such as aluminum–copper alloy [22].
Experimental methods
Metal sample preparation
Sample preparation experiments were carried out by charging a Fe–10 mass%Ni alloy (~ 160 g) in a high-frequency induction furnace with an argon gas protection. A graphite susceptor was installed between a high-purity Al2O3 crucible and induction coil to avoid the induction stirring. The melt composition became homogeneous after 20 min at a constant temperature as 1873 K. After that, the melt was deoxidized using different amount of the Ti addition as 0.03%, 0.1% and 0.2%, respectively. The reader is referred to Ref. [11] for the detailed sampling procedure. The sample time and chemical compositions of each specimen are summarized in Table 1. The dissolved titanium content in the specimens was analyzed using the high-frequency inductively coupled plasma atomic emission spectrometry (ICP-AES). We refer Ref. [23] for more details of the equipment. The total oxygen content in the samples was determined using an inert gas fusion-infrared absorptiometry [24]. The dissolved oxygen content is estimated by (Ototal–Oinsol), where Ototal and Oinsol are total and insoluble oxygen content of the specimen. Oinsol is obtained from the chemical analysis of insoluble Ti content in inclusion. The details can be seen in Ref. [14].
Inclusion characterization
The inclusion characteristics in the metal specimens were observed with a three-dimensional potentiostatic electrolytic extraction (E.E.) method. The detailed experimental parameter for E.E. was described in a previous study [11]. The investigation was performed using a scanning electron microscope (SEM) in combination with an energy-dispersive spectroscopy (EDS) at a magnification of 1000 ×–10,000 ×. The working acceleration voltage of SEM–EDS is between 15 and 20 kV according to the specific resolution, and the beam size of the spot analysis is about 1–2 µm. The calibration material is the pure Fe (= 99.99%). The SEM images were measured using an image software WinROOF®. The equivalent diameter was selected to define the inclusion size.
Results and discussion
Chemical composition of inclusion
Table 2 shows the chemical mapping images of the typical oxide inclusions with different amount of the titanium addition. In order to see a broader image of more particles, the lower magnification of SEM micrographs is provided in Fig. 1. The concentration of Fe and Ti in the inclusions at the sampling time t = 1 min is plotted in Figs. 2, 3 and 4. In the case of Ti = 0.03%, the observed inclusions are the single spheres rather than the clusters. The technical word ‘cluster’ is defined as a group of the agglomerated inclusions. The size range of the single inclusions is from 1.1 up to 6.1 µm, and the Fe/Ti ratio is larger than 5.5, as is shown in Fig. 1. For the case Ti = 0.1% (see Fig. 2), both the single spheres and clusters are found with the size range as 1.1–3.7 µm and 4.5–14.2 µm, respectively. The Fe/Ti ratio is larger than ~ 1.2 in most of the single spheres and smaller than ~ 0.1 in the clusters. When the amount of the titanium addition increases from 0.1 to 0.2%, both the single inclusions (1.1–3.4 µm) and clusters (6.3–12.4 µm) are identified as well, as is shown in Fig. 4. However, the single inclusions have a polygonal shape instead of a spherical shape. Meanwhile, the ratios of Fe/Ti (< 0.04) in both the single inclusions and clusters are quite small.
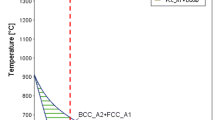
Figure 5 shows a binary phase diagram of the FeO–TiO2 system [20]. Using the above-mentioned Fe/Ti ratio, the mass percentage of the FeO phase in the inclusions is estimated and summarized in Table 3. It can be seen that for the case of Ti = 0.03%, the inclusions with a high FeO content (> 81%) are liquid at the experimental temperature of 1873 K. When the coalescence–collision among the liquid inclusions occurs, the agglomerated droplets will form into one large droplet. Since the turbulent flow is avoided in the experiments, the liquid inclusions can maintain the spherical shape. Analogously, most of the single spheres (FeO > 48%) are liquid at the same temperature in the case of Ti = 0.1%. But the observed clusters are solid due to the low FeO content (< 7%). In the case of Ti = 0.2%, both the single inclusions and clusters with the low FeO content (< 3%) are solid.
FeO–TiO2 phase diagram, adapted from Ref. [25]
Thermodynamic consideration of critical Ti content
Different phases can be precipitated using different titanium amount [16] in the Fe–Ni deoxidation. Suzuki et al. [17] reported that the FeO solubility in the TiO2 phase is much higher than that in the Ti3O5 phase. And the liquid FeO cannot be dissolved into the Ti2O3 phase. The high erosion resistance of the Ti2O3 phase with respect to the liquid FeO was mentioned by Xuan et al. [26] as well. Thus, the thermodynamic data of the TiO2 reaction are selected as an approximation of the TiOx–FeO reaction. The different types of titanium oxide reactions are given by
where K is the equilibrium constant. \( a_{i} \) is the activity of i. The activities of the TiO2, Ti3O5 and Ti2O3 phases equal unity. fO and \( f_{\text{Ti}} \) denote the activity coefficients of the dissolved oxygen and titanium. e j i and r j i are the interaction parameters. The thermodynamic data of the Fe–10%Ni system at 1873 K are calculated based on the experimental results of Dashevskii et al. [16], as is shown in Table 4. Figure 6 shows the equilibrium curve simulated using Eqs. (1)–(5). The [Ti] and [O] contents in the cases of Ti = 0.03%, Ti = 0.1% and Ti = 0.2% are plotted in Fig. 5 as well. It is clear to see the concentrations in all the three cases toward the equilibrium curve with the increased time. Particularly, the concentration in the case of Ti = 0.1% has almost arrived at the equilibrium state after t = 30 min. In the Fe–Ti–O system at a constant temperature as 1873 K, both Hadley et al. [27] and Suzuki et al. [28] identified a boundary content [Ti] ≈ 0.04% between the TiOx–FeO and TiOx precipitation in the equilibrium state. According to chapter 3.1 and Fig. 5, the experimental observation in this work shows a good agreement with the equilibrium boundary in the literature. It indicates that the amount of the titanium addition needs to be smaller than 0.1% to avoid the cluster formation in the melt that includes a total oxygen O = 0.015%.
Theoretical analysis of solid TiOx coalescence behavior
The coalescence behavior of the un-wetting inclusions in the melt depends on different factors. The turbulent flow can facilitate the coalescence efficiency of the inclusions. Meanwhile, the attraction forces among the inclusions can also affect the coalescence efficiency. When an effective coalescence–collision occurs, the sintering of the contacted parts of the inclusions can occur due to the solid-phase diffusion. In this section, the coalescence behavior of the TiOx (x = 1.5–1.67) solid inclusion is theoretically studied from above-mentioned perspectives. As a typical inclusion type, the Al2O3 inclusion is also analyzed for a comparison.
Coalescence coefficient of turbulent collision
The coalescence coefficient, α, of the turbulent collisions is formulated by an expression [29, 30]:
where r is the equivalent radius of the inclusion. ρ = 7000 kg/m3 denotes the density of the melt, ε = 0.01 m2/s3 [31] is the dissipation rate, and µ = 0.006 kg/m s is the viscosity of the melt. A121 is the Hamaker constant of the solid particle in the melt and is given by [32].
where A11 and A22 are the Hamaker constants of the solid and liquid phases. The Hamaker constant of the water and liquid iron equals 4.38 × 10−20 J [33] and 25.3 × 10−19 J [34]. The Hamaker constant of the solid TiOx phase is derived using the surface tension of the water. According to the Fowkes model, the Hamaker constant, A121, between the solid particles in the water is given as [35]
where ds and \( d_{{{\text{H}}_{2} {\text{O}}}} \) denote the interfacial separations of the atomic center at contact for the solid particle and water. d equals 4.0 × 10−10 m for the inorganic materials and 4.3 × 10−10 m for the water at the room temperature. \( \gamma_{\text{s}}^{\text{d}} \) and \( \gamma_{{{\text{H}}_{2} {\text{O}}}}^{\text{d}} \) are the contributions of the London dispersion force to the surface tension of the solid particle and water (= 0.0218 N/m [35]). The London dispersion contribution, \( \gamma_{\text{s}}^{\text{d}} \), is given as [29]
where \( \gamma_{{{\text{H}}_{2} {\text{O}}}} = \, 0.0728 \) N/m is the water surface tension [35]. The contact angle (θ) of both TiO2 (67° ± 2°) and TiOx (x = 1.5–1.67) (18° ± 5°) in contact with the water reported by Kuscer et al. [36] is selected for the calculation. The Hamaker constant of the water equals 4.38 × 10−20 J [33] using the Lifshitz equation. Combining Eqs. (7)–(9), the Hamaker constants, A11, of the TiO2 and TiOx (x = 1.5–1.67) phases are calculated. In the case of the TiO2 phase, the A11 equals 16.3 × 10−20–17.8 × 10−20 J that has a good agreement with the reported data (15.3 × 10−20–17.3 × 10−20 J [37]) using the Lifshitz equation. Thus, the selection of Kuscer et al. [36] data is validated. In this work, the calculated Hamaker constant of the TiOx (x = 1.5–1.67) phase equals A11 = 31.9 × 10−20 J. Using the size r = 1 µm as an example, the coalescence coefficient of the TiOx inclusion α = 0.46 is quite similar to that of the Al2O3 inclusion α = 0.50 [34].
Attraction force due to wettability
When the two inclusions with the low wettability arrive at a certain distance, a void region starts to form between the inclusions by ejecting the melt. The critical distance of the melt ejection is described using Eq. (10) [38].
where γ = 1.75 N/m [35] is the surface tension of the melt. θ is the contact angle between the solid inclusion and melt. P = ρgh is the static pressure of the melt. δ denotes the critical distance. The attraction of the inclusions is driven by the cavity bridge force. The cavity bridge force, FC, describes the sum of the pressure difference (ΔP = 3.86 × 103 Pa [39]) between the void region and melt, and the melt surface tension. It is formulated using the Fisher equation [40]
When the inclusions are in contact with each other, the cavity bridge force can reach maxima, as is shown in Fig. 7. The parameter R is the radius of the void region that is formulated by using the model of Sasai [39]
According to Eqs. (10)–(12), it is clear that for the same inclusion depth in liquid metal (h), the surface tension of liquid metal (γ) and the inclusion size (r), the attraction degree difference between TiOx and Al2O3 inclusion only depends on the contact angle (θ) difference. Consequently, the selection of the contact angle with a high accuracy is quite important for this quantitative comparison.
At the constant temperature as 1873 K, only one contact angle result θ = 128° ± 2° [26] is found for the TiOx (x = 1.5–1.67)/Fe system in the literature. The total oxygen content in the Fe sample after the experiment was smaller than 88 ppm [21]. Humenik and Kingery [41] reported a smaller value θ = 119° at a lower temperature as 1823 K. According to Xuan et al. [26], the interfacial reaction does not occur between the TiOx substrate and the iron. In the case of the non-reactive wetting, the spreading rate of droplet is determined by the viscous flow of the melt [42]. The time length for a small metal droplet with a size of millimeter scale to reach the capillary equilibrium state is shorter than 0.1 s [43]. Due to the high corrosion resistance of the TiOx, the contact angle θ = 128° ± 2° at 1873 K is selected for the analysis.
The results of the contact angle between the Al2O3 substrate and liquid iron/ferrous alloy are quite scattered even though a lot of work [26, 41, 44,45,46,47,48,49,50,51] are reported. Since the spreading behavior of the metal droplet on the Al2O3 substrate depends on both the viscous flow and the interfacial reaction that is given as:
the precipitation of the FeAl2O4 phase at the interface leads to a change on the contact angle. Thus, both the oxygen in the melt and the oxygen partial pressure (PO2) of the protective atmosphere need to be controlled to decrease the effect of the reaction layer. Ogino et al. [44] reported a contact angle θ = 132° at the temperature as 1873 K. The total oxygen in the melt was below 25 ppm after the solidification, and the precipitation of the reaction layer was almost avoided. Kapilashrami et al. [45] reported the same contact angle with the uncertainty of ± 4° at the same temperature. The dissolved oxygen content in the metallic sample after the sessile drop measurement was below the minimum capability of the detection. The same contact angle was also suggested by Poirier et al. [46] using a statistical method.
The contact angle between the TiOx or Al2O3 substrate and the Fe–Ni alloy is not available in the literature. Because of the high similarity of the atomic size between nickel and iron, it is assumed that the contact angle difference between the Fe–10%Ni system and the pure Fe is small. Substituting the above-mentioned contact angles into Eq. (10), the critical distance δ of the TiOx and Al2O3 inclusions is calculated and plotted in Fig. 8. It can be seen that the critical distance increases with the increased size of the inclusion and the decreased depth (h) in the melt. Using the size of 1 µm radius as an example, the critical distance increases from 8.9 µm (h = 1 m) to 336 µm (h = 0.001 m) for the TiOx inclusion and from 9.3 µm (h = 1 m) to 351 µm (h = 0.001 m) for the Al2O3 inclusion. Combining Eqs. (11) and (12), the calculated cavity bridge force of the TiOx inclusion FC = 4.5 × 10−6 N is smaller, compared with that of the Al2O3 inclusion FC = 4.9 × 10−6 N. However, this slight difference can be neglected considering the uncertainty degree of the contact angle measurement.
Agglomeration of the oxide particles at the surface of the molten alloy
Besides the inclusion coalescence in the matrix, its collision behavior at the melt surface is also vital for the removal efficiency of impurity particles. The mechanism of the latter case is different with the former one. Specifically, the attraction of the inclusion at the melt surface is mainly driven by the capillary force (F) [52, 53]. Kralchevsky et al. [54] and Paunov et al. [55] originally derived a capillary force model for this study. In the model, both the energy balance and force balance between the two particles on the melt surface were presented. The change of the capillary interaction energy, ΔW, between the two spherical inclusions with a separation distance L is given by [54, 55].
where Qk and Qk∞ are the effective capillary charges at the separation distance as L and infinity. hk and hk∞ denote the height differences of the meniscus at the separation distance as L and infinity. The subscript k represents inclusions 1 and 2 in an aggregate pair. We refer Ref. [52] for more details of the parameter determination. O(x) is the zero function of the approximation. The parameter q denotes the density ratio between the inclusion and alloy. The attractive capillary force, F, at the different distances (L) gives
The change of the capillary interaction energy ΔW can be calculated by the contributions from wetting, meniscus surface free energy part and gravity. A simplified model to evaluate the capillary force can be expressed as Eq. (16). The details of the equations deviation can be found elsewhere [52, 53].
where Q1 and Q2 are effective capillary charges of inclusions 1 and 2 when the distance between two inclusions is L. The physical parameters including surface tension of liquid metal (γ), density of non-metallic inclusions (ρi (i = 1, 2)), contact angle between liquid melt and inclusion (θ) are used to represent different types of inclusions. The physical parameters data selection can be seen in Ref. [53, 56]. This model has been validated using the confocal laser scanning microscopy, and the results are plotted in Fig. 9. The case of two inclusions in a pair with the same radius of 1 μm is considered as well for attraction analysis at liquid alloy surface. The relative magnitude of the attractive capillary force is as follows: Al2O3 > Ti2O3 > TiO2. It is found that Al2O3 inclusion has a biggest attractive force for the cluster formation. The coalescence potency of Ti2O3 is quite similar to that of the Al2O3 inclusion. For the case of TiOx (x = 1.5–1.67), the exact attraction force is not able to be reported due to the lack of the physical data. However, this force is believed to be between that of the Ti2O3 and TiO2 inclusions according to the influence of the density and contact angle. It is reported that the contact angle and inclusion density are the key factors on influencing the attractive capillary force [53].
Initial sintering of inclusions in melt
According to the theory of the kinetic sintering of the attached solid particles, the relation between the radius of sintering inter-neck (x) and the sintering time is described as [57,58,59]
The sintering time at the initial sintering stage is set as t = 1 min in the current discussion. m and n are the constants related to the different sintering mechanisms. The regime of the sintering mechanism can be determined by plotting the gradient between logarithm of x/r and 2r. The measured gradient of the TiOx case in this work (= −0.6) is the same as that of the Al2O3 case in a previous study [34]. It corresponds to the volume diffusion mechanism (n = 5, m = 2) [57]. A(T) denotes a temperature-dependent function of the solid sintering in an atmosphere medium and is given as [57].
where K is a constant that depends on the geometry of the sample and the diffusion path. According to the measurement, the center–center distance between the two sintered TiOx inclusions is shorter than 2r. Thus, the value of K = 80 is selected based on the research of Kingery and Berg [57]. γs is the surface tension of the solid inclusion, τ3 is the vacancy volume of the solid inclusion, D is the apparent self-diffusion coefficient, and k is Boltzmann’s constant (1.3807 × 10−23 J/K). The above-mentioned parameters are summarized in Table 5. In the case of the inclusions in the melt, the driving force of the external pressure, P0, on the solid sintering needs to be included by using the Coble’s model [60]. Thus, the final set of A(T) is given by.
The external pressure on the inclusions in the melt at the initial sintering stage is expressed as
It is assumed that of the external pressure including two terms. The first term is the static pressure of the melt. The second term is the pressure difference ΔP = 3.86 × 103 Pa [39] between the void region and the melt. The sampling depth in the melt h = 0.1 m is used. In order to obtain the value of the sintered neck radius (x) and inclusions radius (r), more than 60 inter-necks of the TiOx clusters in the sample Ti = 0.2% (t = 1 min) were measured using the image analyzer WinROOF®. Figure 10 shows the schematic illustration of neck radius measurement. The measured parameters of the TiOx inclusions in this work and the Al2O3 inclusions [34] in our previous study are summarized in Table 5. Using the above-mentioned set of equations, the self-diffusion coefficient D = 1.5 × 10−13 m2/s of the TiOx inclusion is obtained. It is about 1.7 times larger than that of the Al2O3 inclusion [34] at a constant temperature as 1873 K.
Conclusions
The focus of the present work is the composition evolution and coalescence behavior of the titanium oxide particles in the Iron-Nickel binary alloy melt. The important conclusions were drawn here.
-
1.
The morphology of the observed inclusion in Fe–10%Ni melt has a tendency to change from a single sphere to an agglomerated cluster with the increase in Ti content.
-
2.
The chemical ratio of Fe/Ti decreases obviously with the increasing Ti addition amount from 0.03 to 0.2%, which leads to the inclusion evolution from the liquid to the solid.
-
3.
Both the experimental results and thermodynamic calculation indicate that the amount of the titanium addition needs to be smaller than Ti = 0.1% to avoid the cluster formation in the melt that includes a total oxygen as 0.015%.
-
4.
The coalescence degree of the TiOx inclusion in the liquid alloy matrix is smaller but closed to that of the Al2O3 inclusion. The similar conclusion can be made for the case of inclusion coalescence at the melt surface, even if the mechanism is the attraction capillary force.
-
5.
The apparent self-diffusion of TiOx for solid sintering is calculated to be about 1.7 times stronger than that of Al2O3. This calculation leads to a statement that TiOx cluster can build up a stable structure more easily compared with Al2O3.
References
Hamzaoui R, Elkedim O, Fenineche N, Gaffet E, Craven J (2003) Structure and magnetic properties of nanocrystalline mechanically alloyed Fe–10% Ni and Fe–20% Ni. Mater Sci Eng A 360(1–2):299–305
Hamzaoui R, Elkedim O, Gaffet E (2004) Milling conditions effect on structure and magnetic properties of mechanically alloyed Fe–10% Ni and Fe–20% Ni alloys. Mater Sci Eng A 381(1–2):363–371
Hamzaoui R, Elkedim O (2013) Magnetic properties of nanocrystalline Fe–10% Ni alloy obtained by planetary ball mills. J Alloys Compd 573:157–162
Li JF, Jie WQ, Yang GC, Zhou YH (2002) Solidification structure formation in undercooled Fe–Ni alloy. Acta Mater 50(7):1797–1807
Zeng T (2017) On the martensitic structure and hardness in as-quenched Fe–Ni alloys. J. Alloys Compd. https://doi.org/10.1016/j.jallcom.2017.08.285
Nakada N (2017) Direct observation of martensitic reversion from lenticular martensite to austenite in Fe–Ni alloy. Mater Lett 187:166–169
Sato H, Zaefferer S (2009) A study on the formation mechanisms of butterfly-type martensite in Fe–30% Ni alloy using EBSD-based orientation microscopy. Acta Mater 57(6):1931–1937
Karasev AV, Suito H (2008) Characteristics of fine oxide particles produced by Ti/M (M = Mg and Zr) complex deoxidation in Fe–10mass% Ni alloy. ISIJ Int 48(11):1507–1516
Pratsinis SE, Kim KS (1989) Particle coagulation, diffusion and thermophoresis in laminar tube flows. J Aerosol Sci 20(1):101–111
Mu W, Dogan N, Coley KS (2018) In situ observation of deformation behavior of chain aggregate inclusions: a case study for Al2O3 at a liquid steel/argon interface. J Mater Sci 53:13203–13215. https://doi.org/10.1007/s10853-018-2557-0
Xuan CJ, Karasev AV, Jönsson PG (2016) Evaluation of agglomeration mechanisms of non-metallic inclusions and cluster characteristics produced by Ti/Al complex deoxidation in Fe–10mass% Ni alloy. ISIJ Int 56(7):1204–1209
Malmberg KJ, Shibata H, Kitamura SY, Jönsson PG, Nabeshima S, Kishimoto Y (2010) Observed behavior of various oxide inclusions in front of a solidifying low-carbon steel shell. J Mater Sci 45(8):2157–2164. https://doi.org/10.1007/s10853-009-3982-x
Mu W, Jönsson PG, Nakajima K (2014) Effect of sulfur content on inclusion and microstructure characteristics in steels with Ti2O3 and TiO2 additions. ISIJ Int 54(12):2907–2916
Karasev A, Suito H (1999) Quantitative evaluation of inclusion in deoxidation of Fe–10 mass pct Ni alloy with Si, Ti, Al, Zr, and Ce. Metall Mater Trans B 30(2):249–257
Sun MK, Jung IH, Lee HG (2008) Morphology and chemistry of oxide inclusions after Al and Ti complex deoxidation. Met Mater Int 14:791–798
Dashevskii VY, Aleksandrov AA, Kanevskii AG, Makarov MA (2010) Deoxidation equilibrium of titanium in the iron–nickel melts. ISIJ Int 50(1):44–52
Suzuki K, Sanbongi K (1975) Equilibrium study on deoxidation of steel with titanium. Trans Iron Steel Inst Jpn 15(12):618–627
Evans EL, Sloman HA (1953) Studies in the deoxidation of iron deoxidation by titanium. J Iron Steel Inst 174:318–324
Chino H, Nakamura Y, Tsunetomi E, Segawa K (1966) The deoxidation with titanium in liquid iron. Tetsu-to-Hagané 52(6):959–966
Kojima Y, Inouye M, Ohi J (1969) Titanoxyd im Gleich- gewicht mit Eisen–Titan–Legierungen bei 1600°C. Arch Eisenhüttenwes 40(9):667–671
Fruehan RJ (1970) Activities in liquid Fe–Al–O and Fe–Ti–O alloys. Metall Trans 1(12):3403–3410
Lombardi A, Mu W, Ravindran C, Dogan N, Barati M (2018) Influence of Al2Cu morphology on the incipient melting characteristics in B206 Al alloy. J Alloys Compd 747:131–139
Sakata K, Suito H (1999) Dispersion of fine primary inclusions of MgO and ZrO2 in Fe–10mass pct Ni alloy and the solidification structure. Metall Mater Trans B 30(6):1053–1063
Inoue R, Suito H (1991) Determination of oxygen in iron-aluminum alloy by inert gas fusion-infrared absorptiometry. Mater Trans JIM 32(12):1164–1169
Macchesney JB, Muan A (1961) Phase equilibria at liquidus temperatures in the system iron oxide-titanium oxide at low oxygen pressures. Am Miner 46:572–582
Xuan CJ, Shibata H, Sukenaga S, Jösson PG, Nakajima K (2015) Wettability of Al2O3, MgO and Ti2O3 by liquid iron and steel. ISIJ Int 55(9):1882–1890
Hadley RL, Derge G (1955) Equilibrium between titanium in liquid iron and titanium oxides. Trans Metall Soc AIME 203:55–60
Suzuki K, Omori Y, Sanbongi K (1967) Deoxidation of steel by titanium. Bull Res Inst Miner Dress Metall 23:137–146
Nakaoka T, Taniguchi S, Matsumoto K, Johansen ST (2001) Particle-size-grouping method of inclusion agglomeration and its application to water model experiments. ISIJ Int 41(10):1103–1111
Lei H, Nakajima K, He JC (2010) Mathematical model for nucleation, Ostwald ripening and growth of inclusion in molten steel. ISIJ Int 50(12):1735–1745
Nakanishi K, Szekely J (1975) Deoxidation kinetics in a turbulent flow field. Trans Iron Steel Inst Jpn 15(10):522–530
Fowkes FM (1964) Attractive forces at interfaces. Ind Eng Chem 56(12):40–52
Visser J (1972) On Hamaker constants: a comparison between Hamaker constants and Lifshitz–van der Waals constants. Adv Colloid Interface Sci 3(4):331–363
Xuan CJ, Karasev AV, Jönsson PG, Nakajima K (2016) Attraction force estimations of Al2O3 particle agglomerations in the melt. Steel Res Int 87(2):1600090. https://doi.org/10.1102/sirn.201600090
Owens DK, Wendt RC (1969) Estimation of the surface free energy of polymers. J Appl Poly Sci 13(8):1741–1747
Kuscer D, Kovac J, Kosec M, Andriesen R (2008) The effect of the valence state of titanium ions on the hydrophilicity of ceramics in the titanium–oxygen system. J Eur Ceram Soc 28(3):577–584
Bergström L (1997) Hamaker constants of inorganic materials. Adv Colloid Interface Sci 70:125–169
Velarde MG and Zeytounian RK (Eds) (2002) Interfacial phenomena and the Marangoni effect, vol 428. Vienna/New York: Springer, p 256
Sasai K (2015) Direct measurement of agglomeration force exerted between alumina particles in molten steel. Tetsu-to-Hagané 101(5):275–283
Fisher RA (1926) On the capillary forces in an ideal soil. J Agric Sci 16(3):492–505
Humenik JRM, Kingery WD (1953) Metal-ceramic interactions: III, surface tension and wettability of metal-ceramic systems. J Am Ceram Soc 37(1):18–23
Eustathopoulos N (1998) Dynamics of wetting in reactive metal/ceramic systems. Acta Mater 46(7):2319–2327
Naidich YV (1981) The wettability of solids by liquid metals. In: Cadenhead DA, Danielli JF (eds), Progress in surface and membrane science, vol 14 Academic Press, New York, pp 353–484
Ogino K, Nogi K, Koshida Y (1973) Effect of oxygen on the wettability of solid oxide with molten iron. Tetsu-to-Hagané 59(10):1380–1387
Kapilashrami E, Jakobsson A, Seetharaman S, Lahiri AK (2003) Studies of the wetting characteristics of liquid iron on dense alumina by the X-ray sessile drop technique. Metal Mater Trans B 34(2):193–199
Poirier DR, Yin HB, Suzuki M, Emi T (1998) Interfacial properties of dilute Fe–O–S melts on alumina substrates. ISIJ Int 38(3):229–238
Jimbo I, Cramb AW (1992) Computer aided interfacial measurements. ISIJ Int 32(1):26–35
Alle BC, Kingery WD (1959) Surface tension and contact angle. Trans Met Soc AIME 215(1):30–37
Takiuchi N, Taniguchi T, Tanaka Y, Shinozaki N, Mukai K (1991) Effects of oxygen and temperature on the surface tension of liquid iron and its wettability of alumina. J Jpn Inst Met 55:180–185
Takiuchi N, Taniguchi T, Shinozaki N, Mukai K (1991) Effects of oxygen on the surface-tension of liquid iron and the wettability of alumina by liquid-iron. J Jpn Inst Met 55:44–49
Nakashima K, Takihira K, Mori K, Shinozaki N (1992) Wettability of Al2O3 substrate by liquid iron-effects of oxygen in liquid iron and purity of Al2O3 substrate. Mater Trans JIM 33(10):918–926
Mu W, Dogan N, Coley KS (2017) Agglomeration of non-metallic inclusions at steel/Ar interface: in-situ observation experiments and model validation. Metall Mater Trans B 48(5):2379–2388
Mu W, Dogan N, Coley KS (2017) Agglomeration of non-metallic inclusions at the steel/Ar interface: model application. Metall Mater Trans B 48(4):2092–2103
Kralchevsky PA, Paunov VN, Denkov ND, Ivanoc IBV, Nagayama KM (1993) Energetical and force approaches to the capillary interactions between particles attached to a liquid-fluid interface. J Colloid Interface Sci 155:420–437
Paunov VN, Kralchevsky PA, Denkov ND, Nagayama K (1993) Lateral capillary forces between floating submillimeter particles. J Colloid Interface Sci 157(1):100–112
Kobatake H, Brillo J (2013) Density and thermal expansion of Cr–Fe, Fe–Ni, and Cr–Ni binary liquid alloys. J Mater Sci 48(14):4934–4941. https://doi.org/10.1007/s10853-013-7274-0
Kingery WD, Berg M (1955) Study of initial stages of sintering solids by viscous flow, evaporation-condensation, and self-diffusion. J Appl Phys 26(10):1205–1212
Kuczynski GG (1949) Self-diffusion in sintering of metallic particles. Trans AIME 185:169–178
Coble RL (1958) Initial sintering of alumina and hematite. J Am Ceram Soc 41(2):55–62
Coble RL (1970) Diffusion models for hot pressing with surface energy and pressure effects as driving forces. J Appl Phys 41:4798–4807
Acknowledgements
CX would like to thank Docent Andrey Karasev and Professor Pär Jönsson at KTH Royal Institute of Technology for the assistance of sample preparation and discussion. WM would like to acknowledge the financial support from the Swedish Foundation for International Cooperation in Research and Higher Education (STINT, No. PT2017-7330).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Xuan, C., Mu, W. Composition evolution and coalescence behavior of titanium oxide particles in Iron-Nickel binary alloy melt. J Mater Sci 54, 8684–8695 (2019). https://doi.org/10.1007/s10853-019-03458-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03458-z