Abstract
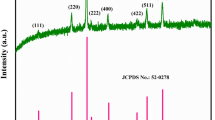
To analyze the impact of preparation routes on the electrochemical behavior of nanoparticles, manganese cobaltite (MnCo2O4) has been synthesized by combustion (MnC-C) and hydrothermal route (MnC-H). The structural properties of synthesized nanoparticles were characterized by X-ray diffractometer studies which confirm the formation of cubic spinel phase with average crystallite size of 26 nm for combustion route prepared and 24 nm for hydrothermal route. The FT-IR spectrum shows two strong bands observed at 651 and 559 cm−1 that are characteristic to stretching vibrations of tetrahedral and octahedral sites of spinel MnCo2O4 compounds. Elemental analysis, oxidation state, and chemical composition of these nanoparticles were examined using X-ray photoelectron spectroscopy. The morphology of synthesized nanoparticles was analyzed by SEM images. Loosely packed flake-like morphology was observed for MnC-H and typical spongy network structure with voids or pores was seen for MnC-C samples. BET analysis reveals the presence of mesopores and micropores in the prepared compounds. Influence of preparation route on capacitor behavior was evaluated by performing electrochemical characterizations such as cyclic voltammetry, chronopotentiometry, and AC impedance analysis. MnC-H exhibits a higher specific capacitance of 671 F g−1 at 5 mV s−1 scan rate compared to 510 F g−1 exhibited by MnC-C electrode material. Excellent capacitance retention of 92 % was demonstrated by MnC-H over 1000 continuous cycling. Results indicate that MnCo2O4 prepared via controlled synthesis conditions (hydrothermal) shows better performance than combustion prepared.







Similar content being viewed by others
References
Yang L, Cheng S, Ding Y, Zhu X, Wang ZL, Liu M (2012) Hierarchical network architectures of carbon fiber paper supported cobalt oxide nanonet for high-capacity pseudocapacitors. Nano Lett 12:321–325. doi:10.1021/nl203600x
Zhou W, Liu J, Chen Tao, Tan KS, Jia X, Luo Z, Cong C, Yang H, Li CM, Ting Y (2011) Fabrication of Co3O4-reduced graphene oxide scrolls for high-performance supercapacitor electrodes. Phys Chem Chem Phys 13:14462–14465. doi:10.1039/c1cp21917k
Djurfors B, Broughton JN, Brett MJ, Ivey DG (2003) Microstructural characterization of porous manganese thin films for electrochemical supercapacitor applications. J Mater Sci 38:4817–4830. doi:10.1023/B:JMSC.0000004401.81145.b6
Deng J-J, Deng J-C, Liu Z-L, Deng H-R, Liu B (2009) Influence of addition of cobalt oxide on microstructure and electrochemical capacitive performance of nickel oxide. J Solid State Electrochem 13:1387–1394. doi:10.1007/s10008-008-0701-5
Chang J-K, Hsieh W-C, Tsai W-T (2008) Effects of the Co content in the material characteristics and supercapacitive performance of binary Mn–Co oxide electrodes. J Alloys Compd 461:667–674. doi:10.1016/j.jallcom.2007.07.092
Babakhani B, Ivey DG (2011) Investigation of electrochemical behavior of Mn–Co doped oxide electrodes for electrochemical capacitors. Electrochim Acta 56:4753–4762. doi:10.1016/j.electacta.2011.03.008
Wu H-Y, Wang H-W (2012) Electrochemical synthesis of nickel oxide nanoparticulate films on nickel foils for high-performance electrode materials of supercapacitors. Int J Electrochem Sci 7:4405–4417
Wang D, Li Y, Wang Q, Wang T (2012) Nanostructured Fe2O3–graphene composite as a novel electrode material for supercapacitors. J Solid State Electrochem 16:2095–2102. doi:10.1007/s10008-011-1620-4
Hinklin TR, Azurdia J, Kim M, Marchal JC, Kumar S, Laine RM (2008) Finding spinel in all the wrong places. Adv Mater 20:1373–1375. doi:10.1002/adma.200702124
Yoon T-J, Kim JS, Kim BG, Yu KN, Cho M-H, Lee J-K (2005) Multifunctional nanoparticles possessing a magnetic motor effect for drug or gene delivery. Angew Chem Int Ed 44:1068–1071. doi:10.1002/anie.200461910
Marco JF, Gancedo RJ, Gracia M, Gautier JL, Rıos EI, Palmer HM, Greaves C, Berry FJ (2001) Cation distribution and magnetic structure of the ferrimagnetic spinel NiCo2O4. J Mater Chem 11:3087–3093. doi:10.1039/b103135j
Martin de Vidales JL, Garcia-Chain P, Rojas R, Vila E, Garcia-Martinez O (1998) Preparation and characterization of spinel-type Mn–Ni–Co–O negative temperature coefficient ceramic thermistors. J Mater Sci 33:1491–1496. doi:10.1023/A:1004351809932
Yokoyama T, Meguro T, Shimada Y, Tatami J, Komeya K, Abe Y (2007) Preparation and electrical properties of sintered oxides composed of Mn1.5Co(0.25+x)Ni(1.25−x)O4 with a cubic spinel structure. J Mater Sci 42:5860–5866. doi:10.1007/s10853-006-1141-1
Li L, Zhang YQ, Liu XY, Shi SJ, Zhao XY, Zhang H, Ge X, Cai GF, Gu CD, Wang XL, Tu JP (2014) One-dimension MnCo2O4 nanowire arrays for electrochemical energy storage. Electrochim Acta 116:467–474. doi:10.1016/j.electacta.2013.11.081
Gomez J, Kalu EE (2013) High-performance binder-free Co–Mn composite oxide supercapacitor electrode. J Power Sources 230:218–224. doi:10.1016/j.jpowsour.2012.12.069
Kim SW, Lee HW, Muralidharan P, Seo DH, Yoon WS, Kim D, Kang K (2011) Electrochemical performance and exsitu analysis of ZnMn2O4 nanowires as anode materials for lithium rechargeable batteries. Nano Res 4:505–510. doi:10.1007/s12274-011-0106-0
Jiang H, Ma J, Li CZ (2012) Hierarchical porous NiCo2O4 nanowires for high-rate supercapacitors. Chem Commun 48:4465–4467. doi:10.1039/C2CC31418E
Wang Z, Zhang X, Li Y, Liu ZT, Hao ZP (2013) Synthesis of graphene–NiFe2O4 nanocomposites and their electrochemical capacitive behavior. J Mater Chem A 1:6393–6399. doi:10.1039/C3TA10433H
Karthikeyan K, Kalpana D, Renganathan NG (2009) Synthesis and characterizationof ZnCo2O4 nanomaterial for symmetric supercapacitor applications. Ionics 15:107–110. doi:10.1007/s11581-008-0227-y
Naveen AN, Selladurai S (2014) Investigation on physiochemical properties of Mn substituted spinel cobalt oxide for supercapacitor applications. Electrochim Acta 125:404–414. doi:10.1016/j.electacta.2014.01.161
Prasad KR, Miura N (2004) Electrochemically synthesized MnO2-based mixed oxides for high performance redox supercapacitors. Electrochem Commun 6:1004–1008. doi:10.1016/j.elecom.2004.07.017
Bordeneuve H, Tenailleau C, Guillemet-Fritsch S, Smith R, Suard E, Rousset A (2010) Structural variations and cation distributions in Mn3−xCoxO4 (0 < x < 3) dense ceramics using neutron diffraction data. Solid State Sci 12:379–386. doi:10.1016/j.solidstatesciences.2009.11.018
Gautier JL, Rios E, Gracia M, Marco JF, Gancedo JR (1997) Characterisation by X-ray photoelectron spectroscopy of thin MnxCo3−xO4 (1 ≥ x ≥ 0) spinel films prepared by low-temperature spray pyrolysis. Thin Solid Films 311:51–57. doi:10.1016/S0040-6090(97)00463-X
Blasse G (1963) Superexchange in the spinel structure. Some magnetic properties of oxides M2+Co2O4 and M2+Rh2O4 with spinel structure. Philips Res Rep Suppl 18:383
Kolomiets BT, Sheftel J, Kurlina E (1957) Electrical properties of some compound oxide semiconductors. Sov Phys Tech Phys 2:40–58
Wickham DG, Croft W (1958) Crystallographic and magnetic properties of several spinels containing trivalent ja-1044 manganese. J Phys Chem Solids 7:351
Gubanov A (1957) Sov Phys I.C.S. Technol Phys 2:47
Sutka A, Mezinskis G (2012) Sol–gel auto-combustion synthesis of spinel-type ferrite nanomaterials. Front Mater Sci 6:128–141. doi:10.1007/s11706-012-0167-3
Verma S, Joshi HM, Jagadale T, Chawla A, Chandra R, Ogale S (2008) Nearly monodispersed multifunctional NiCo2O4 spinel nanoparticles: magnetism, infrared transparency and radiofrequency absorption. J Phys Chem C 112:15106–15112. doi:10.1021/jp804923t
Hayashi H, Hakuta Y (2010) Hydrothermal synthesis of metal oxide nanoparticles in supercritical water. Materials 3:3794–3817. doi:10.3390/ma3073794
Zhang X, Shi W, Zhu J, Zhao W, Ma J, Mhaisalkar S, Maria TL, Yang Y, Zhang H, Hng HH, Yan Q (2010) Synthesis of porous NiO nanocrystals with controllable surface area and their application as supercapacitor electrodes. Nano Res 3:643–652. doi:10.1007/s12274-010-0024-6
Meher SK, Rao GR (2011) Ultralayered Co3O4 for high-performance supercapacitor applications. J Phys Chem C 115:15646–15654. doi:10.1021/jp201200e
Du W, Liu R, Jiang Y, Lu Q, Fan Y, Gao F (2013) Facile synthesis of hollow Co3O4 boxes for high capacity supercapacitor. J Power Sources 227:101–105. doi:10.1016/j.jpowsour.2012.11.009
Duan BR, Cao Q (2012) Hierarchically porous Co3O4 film prepared by hydrothermal synthesis method based on colloidal crystal template for supercapacitor application. Electrochim Acta 64:154–161. doi:10.1016/j.electacta.2012.01.004
Tholkappiyan R, Vishista K (2014) Influence of lanthanum on the optomagnetic properties of zinc ferrite prepared by combustion method. Physica B 448:177–183. doi:10.1016/j.physb.2014.04.022
Subramanian V, Hall SC, Smith PH, Rambabu B (2004) Mesoporous anhydrous RuO2 as a supercapacitor electrode material. Solid State Ionics 175:511–515. doi:10.1016/j.ssi.2004.01.070
Vu D, Li X, Li Z, Wang C (2013) Phase-structure effects of electrospun TiO2 nanofiber membranes on As(III) adsorption. J Chem Eng Data 58:71–77. doi:10.1021/je301017q
Luo W, Hu X, Sun Y, Huang Y (2012) Electrospun porous ZnCo2O4 nanotubes as a high-performance anode material for lithium-ion batteries. J Mater Chem 22:8916–8921. doi:10.1039/c2jm00094f
Rojas RM, Vila E, García O, de Vidales JLM (1994) Thermal behaviour and reactivity of manganese cobaltites MnxCo3−xO4(0.0 ≤ x ≤ 1.0) obtained at low temperature. J Mater Chem 4:1635–1639. doi:10.1039/jm9940401635
Tan BJ, Klabunde KJ, Sherwood PMA (1991) XPS studies of solvated metal atom dispersed (SMAD) catalysts. Evidence for layered cobalt-manganese particles on alumina and silica. J Am Chem Soc 113:855–861. doi:10.1021/ja00003a019
Xia X-H, Tu J-P, Wang X-L, Gua C-D, Zhao X-B (2011) Mesoporous Co3O4 monolayer hollow-sphere array as electrochemical pseudocapacitor material. Chem Commun 47:5786–5788. doi:10.1039/C1CC11281C
Jiang H, Zhao T, Yan C, Ma J, Li C (2010) Hydrothermal synthesis of novel Mn3O4 nano-octahedrons with enhanced supercapacitors performances. Nanoscale 2:2195–2198. doi:10.1039/C0NR00257G
Kong L-B, Lu C, Liu M-C, Luo Y-C, Kang L, Li X, Walsh FC (2014) The specific capacitance of sol–gel synthesised spinel MnCo2O4 in analkaline electrolyte. Electrochim Acta 115:22–27. doi:10.1016/j.electacta.2013.10.089
Ding R, Qi L, Wang H (2012) A facile and cost-effective synthesis of mesoporous NiCo2O4 nanoparticles and their capacitive behavior in electrochemical capacitors. J Solid State Electrochem 16:3621–3633. doi:10.1007/s10008-012-1798-0
Meher SK, Rao GR (2011) Effect of microwave on the nanowire morphology, optical, magnetic, and pseudocapacitance behavior of Co3O4. J Phys Chem C 115:25543–25556. doi:10.1021/jp209165v
Wei T-Y, Chen C-H, Chang K-H, Lu S-Y, Hu C-C (2009) Cobalt oxide aerogels of ideal supercapacitive properties prepared with an epoxide synthetic route. Chem Mater 21:3228–3233. doi:10.1021/cm9007365
Zhang Y, Luo L, Zhang Z, Ding Y, Liu S, Deng D, Zhao H, Chen Y (2014) Synthesis of MnCo2O4 nanofibers by electrospinning and calcination: application for a highly sensitive non-enzymatic glucose sensor. J Mater Chem B 2:529–535. doi:10.1039/C3TB21288B
Srinivasan V, Weidner JW (2002) Capacitance studies of cobalt oxide films formed via electrochemical precipitation. J Power Sources 108:15–20. doi:10.1016/S0378-7753(01)01012-6
Acknowledgements
The authors thank Mr. S. Venkatesh for XPS measurement at MNCF, Centre for Nano Science and Engineering (CeNSE), IISc Bangalore, India and also thank to Mr. S Karuppusamy, Research scholar for FT-IR measurement at Department of Physics, Anna University, Chennai-25, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tholkappiyan, R., Naveen, A.N., Sumithra, S. et al. Investigation on spinel MnCo2O4 electrode material prepared via controlled and uncontrolled synthesis route for supercapacitor application. J Mater Sci 50, 5833–5843 (2015). https://doi.org/10.1007/s10853-015-9132-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9132-8