Abstract
Isorhamnetin has been reported to have anti-inflammatory, anti-oxidative, and anti-proliferative effects. The aim of this study was to investigate the protective effect of isorhamnetin on lipopolysaccharide (LPS)-induced acute lung injury (ALI) in mice by inhibiting the expression of cyclooxygenase-2 (COX-2). The effects of isorhamnetin on LPS-induced lung pathological damage, wet/dry ratios and the total protein level in bronchoalveolar lavage fluid (BALF), inflammatory cytokine release, myeloperoxidase (MPO) and superoxide dismutase (SOD) activities, and malondialdehyde (MDA) level were examined. In addition, the COX-2 activation in lung tissues was detected by Western blot. Isorhamnetin pretreatment improved the mice survival rates. Moreover, isorhamnetin pretreatment significantly attenuated edema and the pathological changes in the lung and inhibited protein extravasation in BALF. Isorhamnetin also significantly decreased the levels of inflammatory cytokines in BALF. In addition, isorhamnetin markedly prevented LPS-induced oxidative stress. Furthermore, isorhamnetin pretreatment significantly suppressed LPS-induced activation of COX-2. Isorhamnetin has been demonstrated to protect mice from LPS-induced ALI by inhibiting the expression of COX-2.
Similar content being viewed by others
INTRODUCTION
Acute respiratory distress syndrome (ARDS), an indication of acute lung injury (ALI), is highly associated with sepsis [1, 2], pneumonias [3], and severe acute respiratory syndrome (SARS) [4]. Despite significant advances in intense care research and diverse therapeutic trials made in the past few decades, ALI remains a severe disease and still presents a high mortality rate of approximately 40 % [5, 6]. Therefore, a novel effective therapeutic agent is urgently required.
Cyclooxygenase-2 (COX-2) plays an important role in the mediation of the inflammatory state. Two isoforms of COX have been described: COX-1, which is constitutively expressed in most tissues, and COX-2, which can be induced in certain cell types by inflammatory stimuli [7, 8]. It has been proven that COX-2 could be activated and increased by pro-inflammatory cytokines and further aggravates the inflammatory immune response in lung injury [9]. COX-2 expression can also be upregulated in the models of lipopolysaccharide (LPS)-induced ALI in mice.
Isorhamnetin (molecular formula: C16H12O7, Fig. 1), a flavonol aglycone, isolated from the traditional Chinese medicine Hippophae rhamnoides L., was frequently used in traditional medicine to prevent and treat diverse diseases [10, 11]. Previous study has illustrated the protective effect of isorhamnetin on carrageenan-induced paw swelling in hind [12]. In addition, it has been reported to have anti-proliferative and anti-viral effects [11, 13]. However, whether isorhamnetin can protect lungs suffering from LPS instillation remains unclear.
We, therefore, for the first time, explored the effects of isorhamnetin on LPS-induced ALI in mice and investigated the possible mechanism.
MATERIALS AND METHODS
Preparation of Isorhamnetin
The preparation of isorhamnetin is according to the SFDA’s statement. Isorhamnetin was isolated from Oenanth e javanica as previously reported [12, 14]. Briefly, 10 kg of air-dried stems and leaves of O. javanica was powdered and then immersed into MeOH for three times. The methanolic extract was then immersed in water and partitioned successively with CHCl3 and n-BuOH. The n-BuOH fraction was eluted with CHCl3. The CHCl3-MeOH (25:1) fraction was concentrated, combined, and evaporated in a rotor evaporator at controlled temperature (50–60 °C) and then shade dried. Thus, the isorhamnetin was obtained. Its chemical structure was confirmed by HPLC-ESI-MS and NMR spectroscopy as previously described [12].
Ethics Statement
All the experimental procedures involving animals were approved by the Ethics Committee of Tangdu Hospital, The Fourth Military Medical University (Permit Number: 2013524). All animal work was carried out in accordance with national and international guidelines to minimize suffering to animals.
Animals and Reagents
Sixty male BALB/C mice weighing 20–25 g were purchased from the Animal Center of The Fourth Military Medical University (Xi’an, China). All animals were allowed to take food and tap water ad libitum.
LPS (O55:B5) was obtained from Sigma Chemical Company (St. Louis, MO). Enzyme-linked immunosorbent assay kits for detecting mouse tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) were purchased from R&D Corporation (Minneapolis, MN). Myeloperoxidase (MPO) kit, malondialdehyde (MDA) kit, and superoxide dismutase (SOD) kit were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Rabbit polyclonal antibodies specific for COX-2 and β-actin were obtained from the Wuhan Boster Biological Technology, Ltd. (Wuhan, China). Cell lysis buffer and bicinchoninic acid (BCA) protein assay kit were purchased from Beyotime Institute of Biotechnology (Beijing, China).
Design
Animals were anesthetized with intraperitoneal pentobarbital (50 mg/kg). LPS (3 mg/kg) was instilled intratracheally to induce ALI. Animals were randomly allocated into four groups, i.e., control (saline-treated; n = 15), isorhamnetin (60 mg/kg)-treated (n = 15), LPS-treated (n = 15), and isorhamnetin (60 mg/kg) + LPS-treated (n = 15). Isorhamnetin was given with an intragastric administration 1 h before LPS administration. The animals in the control group received the same treatments of saline. The mice were sacrificed 6 h after LPS administration.
Survival Studies
For the observation of mortality rates, the male BALB/C mice were subjected to LPS (50 mg/kg). They were randomized into three groups (n = 10 per group): control (saline-treated), LPS-treated, and isorhamnetin (60 mg/kg) + LPS-treated. The control group received the same volume of saline. The mortality of the mice in each treated group was recorded every 12 h for 3 days after the LPS injection.
BALF
After animals were killed, a median sternotomy allowed for exposure of both the lungs. The trachea was exposed and an intravenous infusion needle was inserted. After ligating the hilum of the right lung, the left lung was lavaged three times with 0.5 ml of ice-cold phosphate-buffered saline. The lavage fluid was pooled for each animal and then centrifuged at 1000×g for 5 min at 4 °C. The cell-free supernatants were harvested for total protein analysis using the BCA protein assay kit. The concentrations of TNF-α and IL-1β in the supernatants were determined using enzyme-linked immunosorbent assay kits. All procedures were done according to the manufacturer’s instructions.
Histopathologic Analysis
The superior lobe of the right lung was harvested at 6 h after LPS administration and fixed with an intratracheal instillation of 1 ml buffered formalin (10 %, pH 7.2). The lobe was further fixed in 10 % neutral buffered formalin for 48 h at 4 °C. The tissues were embedded in paraffin wax. Sections approximately 5 μm thick were stained with hematoxylin and eosin using a standard protocol.
Estimation of Pulmonary Edema
As an index of lung edema, the amount of extravascular lung water was calculated. The middle lobe of the right lung was excised and the wet weight was recorded. The lobe was then placed in an incubator at 80 °C for 24 h to obtain the dry weight. And the wet/dry weight ratios were calculated by dividing the wet weight by the dry weight.
Oxidative Stress In Vivo
Lung tissues were frozen in liquid nitrogen and then homogenized in PBS. The MPO activities in the lung homogenates were examined by using a MPO determination kit. The rest of the homogenate was centrifuged at 2000g for 10 min at 4 °C. The supernatants were used to detect the levels of MDA and SOD according to the manufacturer’s instruction.
Western Blotting
Lung tissue samples were harvested and frozen in liquid nitrogen immediately until homogenization. Tissue samples were homogenized in cell lysis buffer. After centrifugation (12,000×g, 10 min, 4 °C), supernatants were aspirated. Protein concentrations were determined by BCA protein assay kit. Samples were separated on a denaturing 12 % polyacrylamide gel and transferred to a nitrocellulose membrane. COX-2 proteins were detected by chemiluminescence using a rabbit polyclonal antibody according to the manufacturer’s instructions.
Statistical Analysis
Survival rates were compared by Kaplan–Meier log-rank test. Data were expressed as mean ± standard deviation. All statistical analysis was performed with SPSS 17.0 software package (SPSS Inc, Chicago, IL). Statistically significant differences between groups were determined by ANOVA followed by Tukey’s test. Results were considered statistically significant if P values were <0.05.
RESULTS
The Effects of Isorhamnetin on LPS-Induced Mortality in Mice
As shown in Fig. 2, following LPS, isorhamnetin significantly reduced the death of mice induced by LPS. About 85 % of mice in the LPS-treated group died in less than 72 h. However, isorhamnetin + LPS-treated animals displayed a significantly longer survival time. The 72 h survival rate in the isorhamnetin + LPS-treated group was significantly higher, compared to that in the LPS-treated group (P < 0.05).
Histopathological Changes in LPS-Induced ALI Mice
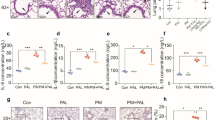
As shown in Fig. 3a–e, lung tissues from the control and isorhamnetin-treated groups showed a normal structure and no histopathological changes under a light microscope (Fig. 3a, b). In the LPS-treated group, the lungs stained with hematoxylin–eosin indicated widespread alveolar wall thickness caused by edema, severe hemorrhage in the alveolus, alveolus collapse, and obvious inflammatory cell infiltration (Fig. 3c). In the isorhamnetin + LPS-treated group, the histopathological changes of lung were minor compared with those in the LPS-treated group, especially in inflammatory cell infiltration (Fig. 3d).
The effects of isorhamnetin on the lung histopathological changes (hematoxylin–eosin stain, ×200). The lung tissues were stained with hematoxylin and eosin to reveal histopathological changes for the following groups: a control, b isorhamnetin-treated, c LPS-treated, and d isorhamnetin + LPS-treated. e Lung injury scores. Data are expressed as mean ± standard deviation. *P < 0.05 vs. the control and isorhamnetin-treated groups; #P < 0.05 vs. the LPS-treated group.
Effects of Isorhamnetin on Pulmonary Edema
As shown in Fig. 4, compared with the control and isorhamnetin-treated groups, the lung wet/dry weight ratios (Fig. 4a) and total protein in bronchoalveolar lavage fluid (BALF) (Fig. 4b) were significantly increased after LPS administration. The increase of the lung wet/dry weight ratios and total protein in BALF were significantly reduced by isorhamnetin administration (P < 0.05).
Effects of Isorhamnetin on Concentrations of TNF-α and IL-1β in BALF
As shown in Fig. 5, the concentrations of TNF-α and IL-1β in BALF were significantly increased after LPS administration. Isorhamnetin pretreatment efficiently reduced the production of TNF-α and IL-1β (Fig. 5a, b).
Isorhamnetin Reduced MPO Activity and MDA Formation and Enhanced SOD Activity in LPS-Induced ALI Mice
As shown in Fig. 6a, after LPS administration, the MPO activity in lung tissues was significantly increased compared with the control and isorhamnetin-treated groups. However, isorhamnetin pretreatment markedly decreased the MPO activity.
As shown in Fig. 6b, c, significant elevation in MDA content and decrease in SOD activity were observed in the lung tissues of the LPS-treated group when compared with controls. Treatment with isorhamnetin partly prevented marked elevation in MDA levels and decrease in SOD activities.
Effects of Isorhamnetin on the Expression of COX-2 in LPS-Induced ALI Mice
As shown in Fig. 7, after LPS administration, the expression of COX-2 in lung tissues markedly increased. However, the pretreatment of isorhamnetin significantly suppressed LPS-induced activation of COX-2. There were no significant changes in the expression of COX-2 in the control and isorhamnetin-treated groups.
Effects of isorhamnetin on the expression of COX-2 in lung tissues. a A representative Western blot showing the expression of COX-2 in lung tissues in different groups and mean ± standard deviation. b COX-2 optical densitometry from different groups. Data are expressed as mean ± standard deviation. *P < 0.05 vs. the control and isorhamnetin-treated groups; #P < 0.05 vs. the LPS-treated group.
DISCUSSION
In the present study, we have demonstrated that isorhamnetin pretreatment could significantly improve survival in a mouse model of ALI induced by LPS. We have also identified some of the underlying mechanisms for this protective effect: amelioration of ALI, inhibition of MPO and MDA in the lung, and reduction of TNF-α and IL-1β in BALF. To the best of our knowledge, this is the first study that evaluates the effect of isorhamnetin on LPS-induced ALI via the COX-2 pathways.
Although some promising pharmacological therapies have been studied in patients with ALI and ARDS, none of these treatments effectively reduced mortality [15].
Isorhamnetin is a traditional Chinese medicine that displays various properties, such as anti-inflammatory, anti-oxidative, and anti-proliferative effects [10–14]. Recent data showed that isorhamnetin administration inhibited carrageenan-induced acute inflammatory response in rats. In addition, studies suggest that isorhamnetin inhibited JNK and AKT/IKKα/β activation, leading to NF-κB inactivation, which might contribute to the inhibition of the acute inflammatory response [12]. It is well known that long-term treatment with LPS leads to cell death via reactive oxygen species/reactive nitrogen species (ROS/RNS) accumulation in Raw264.7 cells. However, Yang et al. found that isorhamnetin inhibits LPS-induced ROS/RNS generations and subsequent cell death [14].
We explored the underlying mechanism of isorhamnetin protection against ALI/ARDS. It has been reported that a network of pro-inflammatory cytokines, such as TNF-α and IL-1β, has a close relationship with the ALI model [16, 17]. We found that isorhamnetin pretreatment restrained all of the LPS-induced increases in TNF-α and IL-1β in vivo. LPS significantly increased the production of intracellular pro-inflammatory cytokines, and this was inhibited by isorhamnetin. This finding suggested that isorhamnetin could reduce the production of pro-inflammatory cytokines after LPS stimulation.
Neutrophils are also an important component of the inflammatory response that characterizes ALI and are considered to be the final effector cell responsible for lung injury, due to their ability to express multiple cytotoxic products [18, 19]. MPO is a major constituent of neutrophil cytoplasmic granules [20]. In the current study, we found the MPO activity increased evidently in lung tissues after LPS exposure. As expected, we found that isorhamnetin pretreatment significantly reduced the MPO activity in lung tissues.
In addition, histopathologic analysis also showed that isorhamnetin pretreatment significantly reduced the neutrophil infiltration in lung tissues. These findings suggest that the protective effects of isorhamnetin on ALI may be associated with its attenuation of proliferation and migration of neutrophils into lung tissue. As the pulmonary edema is a major characteristic of ALI, we evaluated the lung wet/dry weight ratio to quantify the magnitude of pulmonary edema. As expected in this study, the lung wet/dry weight ratio increased markedly in mice treated with LPS and could be significantly decreased by isorhamnetin pretreatment.
MDA is a lipid peroxidation product which has been used as an index of induced oxidative membrane damage [21, 22]. In the present work, we measured MDA concentration in the lungs of LPS-challenged mice and detected lower MDA contents in the isorhamnetin + LPS-treated animals. On the contrary, the activity of SOD, an endogenous free radical-scavenging agent which can eliminate oxyradical, was also examined [23]. The data demonstrated that SOD activities increased in mice pretreated with isorhamnetin when compared with those in the LPS-treated group.
The COX enzymes (COX-1 and COX-2) could convert arachidonic acid to prostanoid, which are involved in the development of both early and late phases of the pathogenesis of ALI [24]. Therefore, inhibiting the increase of COX-2 activity induced by LPS is expected to attenuate the ALI [25, 26]. This is supported by results of the present study demonstrating that isorhamnetin pretreatment inhibited the COX-2 expression.
However, pretreatment of drugs was not often possible clinically, which was a limitation of this study. In the further study, we will evaluate the protective effect of delayed isorhamnetin treatment on ALI.
In conclusion, we have revealed for the first time that isorhamnetin pretreatment improves survival and attenuates ALI in a mouse model of LPS by decreasing neutrophil trapping, creating an anti-inflammatory environment, and minimizing oxidative damage. The potential mechanism of this action may involve the inhibitions of COX-2 expression. Our study suggests that isorhamnetin might be useful in the therapy of ALI.
References
Dreyfuss, D., and J.D. Ricard. 2005. Acute lung injury and bacterial infection. Clinics in Chest Medicine 26: 105–112.
Baumgarten, G., P. Knuefermann, H. Wrigge, et al. 2006. Role of Toll-like receptor 4 for the pathogenesis of acute lung injury in Gram-negative sepsis. European Journal of Anaesthesiology 23: 1041–1048.
Chen, H.I., S.J. Kao, D. Wang, et al. 2003. Acute respiratory distress syndrome. Journal of Biomedical Science 10: 588–592.
Gu, J., and C. Korteweg. 2007. Pathology and pathogenesis of severe acute respiratory syndrome. American Journal of Pathology 170: 1136–1147.
Rubenfeld, G.D., E. Caldwell, E. Peabody, et al. 2005. Incidence and outcomes of acute lung injury. New England Journal of Medicine 353: 1685–1693.
Zambon, M., and J.L. Vincent. 2008. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 133: 1120–1127.
Hla, T., and K. Neilson. 1992. Human cyclooxygenase-2 cDNA. Proceedings of the National Academy of Sciences of the United States of America 89: 7384–7388.
Masferrer, J.L., B.S. Zweifel, P.T. Manning, et al. 1994. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proceedings of the National Academy of Sciences of the United States of America 91: 3228–3232.
Ni, Y.F., T. Jiang, Q.S. Cheng, et al. 2012. Protective effect of magnolol on lipopolysaccharide-induced acute lung injury in mice. Inflammation 35(6): 1860–1866.
Park, J.C., H.S. Young, Y.B. Yu, et al. 1995. Isorhamnetin sulphate from the leaves and stems of Oenanthe javanica in Korea. Planta Medica 61: 377–378.
Apers, S., Y. Huang, S. Van Miert, et al. 2002. Characterisation of new oligoglycosidic compounds in two Chinese medicinal herbs. Phytochemical Analysis 13: 202–206.
Yang, J.H., S.C. Kim, B.Y. Shin, et al. 2013. O-Methylated flavonol isorhamnetin prevents acute inflammation through blocking of NF-κB activation. Food and Chemical Toxicology 59: 362–372.
Teng, B.S., Y.H. Lu, Z.T. Wang, et al. 2006. In vitro anti-tumor activity of isorhamnetin isolated from Hippophae rhamnoides L. against BEL-7402 cells. Pharmacological Research 54: 186–194.
Yang, J.H., B.Y. Shin, J.Y. Han, et al. 2014. Isorhamnetin protects against oxidative stress by activating Nrf2 and inducing the expression of its target genes. Toxicology and Applied Pharmacology 274: 293–301.
Sprung, C.L., D. Annane, D. Keh, et al. 2008. Hydrocortisone therapy for patients with septic shock. New England Journal of Medicine 358: 111–124.
Luo, Y., B. Zhang, D.Q. Xu, et al. 2011. Protective effect of bicyclol on lipopolysaccharide-induced acute lung injury in mice. Pulmonary Pharmacology & Therapeutics 24: 240–246.
Wan, L.M., L. Tan, Z.R. Wang, et al. 2013. Preventive and therapeutic effects of Danhong injection on lipopolysaccharide induced acute lung injury in mice. Journal of Ethnopharmacology 149: 352–359.
Abraham, E. 2003. Neutrophils and acute lung injury. Critical Care Medicine 31: 195–199.
Parsey, M.V., R.M. Tuder, and E. Abraham. 1998. Neutrophils are major contributors to intraparenchymal lung IL-1beta expression after hemorrhage and endotoxemia. Journal of Immunology 160: 1007–1013.
Yang, B., Y.-F. Ni, W.-C. Wang, et al. 2015. Melatonin attenuates intestinal ischemia reperfusion-induced lung injury in rats by upregulating N-myc downstream-regulated gene 2. Journal of Surgical Research 194(1): 273–280.
Kilic, T., H. Parlakpinar, A. Polat, et al. 2014. Protective and therapeutic effect of molsidomine on bleomycin-induced lung fibrosis in rats. Inflammation 37(4): 1167–1178.
Lee, J.H., Y.H. Jo, K. Kim, et al. 2013. Effect of N-acetylcysteine (NAC) on acute lung injury and acute kidney injury in hemorrhagic shock. Resuscitation 84: 121–127.
Macarthur, H., T.C. Westfall, D.P. Riley, et al. 2009. Inactivation of catecholamines by superoxide gives new insights on the pathogenesis of septic shock. Proceedings of the National Academy of Sciences of the United States of America 97: 9753–9758.
Anderson, F.L., W. Jubiz, T.J. Tsagaris, et al. 1975. Endotoxin-induced prostaglandin E and F release in dogs. American Journal of Physiology 228: 410–414.
Fan, G.W., Y. Zhang, X. Jiang, et al. 2013. Anti-inflammatory activity of baicalein in LPS-stimulated RAW264.7 macrophages via estrogen receptor and NF-κB-dependent pathways. Inflammation 36(6): 1584–1591.
Wang, T., M. Guo, X. Song, et al. 2014. Stevioside plays an anti-inflammatory role by regulating the NF-κB and MAPK pathways in S. aureus-infected mouse mammary glands. Inflammation 37(5): 1837–1846.
Acknowledgments
The studies were supported by the National Natural Science Foundation of China (81070062) and the Military Medical Scientific Research Items of China (13MA228). The authors are grateful to Ms. Xue-Jiao Wang, Prof. Xiao-Fei Li, Prof. Xiao-Long Yan, Prof. Zhi-Pei Zhang, Dr. Lin Zhou, and Dr. Hong Zhang for providing excellent technical assistance.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Bo Yang, Xiao-Ping Li, and Rong Wang contributed equally to this work and should be regarded as co-first authors.
Rights and permissions
About this article
Cite this article
Yang, B., Li, XP., Ni, YF. et al. Protective Effect of Isorhamnetin on Lipopolysaccharide-Induced Acute Lung Injury in Mice. Inflammation 39, 129–137 (2016). https://doi.org/10.1007/s10753-015-0231-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0231-0