Abstract
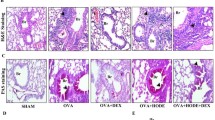
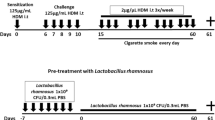
Neutrophilic asthma is generally defined by poorly controlled symptoms and high levels of neutrophils in the lungs. Short-chain fatty acids (SCFAs) are proposed as nonpharmacological therapy for allergic asthma, but their impact on the neutrophilic asthma lacks evidence. SCFAs regulate immune cell responses and impact the inflammasome NLRP3, a potential pharmacological target for neutrophilic asthma. Here, we explored the capacity of SCFAs to mitigate murine-induced neutrophilic asthma and the contribution of NLRP3 to this asthma. The objective of this study is to analyze whether SCFAs can attenuate lung inflammation and tissue remodeling in murine neutrophilic asthma and NLRP3 contribution to this endotype. Wild-type (WT) C57BL6 mice orotracheally received 10 μg of HDM (house dust mite) in 80 μL of saline on days 0, 6–10. To explore SCFAs, each HDM group received 200 mM acetate, propionate, or butyrate. To explore NLRP3, Nlrp3 KO mice received the same protocol of HDM. On the 14th day, after euthanasia, bronchoalveolar lavage fluid (BALF) and lungs were collected to evaluate cellularity, inflammatory cytokines, and tissue remodeling. HDM group had increased BALF neutrophil influx, TNF-α, IFN-γ, IL-17A, collagen deposition, and mucus secretion compared to control. SCFAs distinctively attenuate lung inflammation. Only features of tissue remodeling were Nlrp3-dependent such as collagen deposition, mucus secretion, active TGF-β cytokine, and IMs CD206+. SCFAs greatly decreased inflammatory cytokines and tissue remodeling. Only tissue remodeling was dependent on NLRP3. It reveals the potential of SCFAs to act as an additional therapy to mitigate neutrophilic asthma and the NLRP3 contribution to asthma.







Similar content being viewed by others
AVAILABILITY OF DATA AND MATERIALS
All data are available upon reasonable request to the corresponding author.
References
Reddel, H.K., L.B. Bacharier, E.D. Bateman, C.E. Brightling, G.G. Brusselle, R. Buhl, et al. 2022. Global initiative for asthma strategy 2021: executive summary and rationale for key changes. Journal of Allergy and Clinical Immunology: In Practice [Internet] 10: S1–S18. Available from https://linkinghub.elsevier.com/retrieve/pii/S2213219821010643.
Chung, K.F., S.E. Wenzel, J.L. Brozek, A. Bush, M. Castro, P.J. Sterk, et al. 2014. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. European Respiratory Journal [Internet] 43: 343–73. Available from http://erj.ersjournals.com/lookup/doi/10.1183/09031936.00202013.
López-Tiro, J., A. Contreras-Contreras, M.E. Rodríguez-Arellano, and P. Costa-Urrutia. 2022. Economic burden of severe asthma treatment: a real-life study. World Allergy Organization Journal [Internet] 15: 100662. Available from https://linkinghub.elsevier.com/retrieve/pii/S1939455122000382.
Ding, B., S. Chen, D. Srivastava, A. Quinton, W. Cook, A. Papi, et al. 2023. Symptom burden, health status, and productivity in patients with uncontrolled and controlled severe asthma in novelty. J Asthma Allergy [Internet] 16: 611–624. Available from https://www.dovepress.com/symptom-burden-health-status-and-productivity-in-patients-with-uncontr-peer-reviewed-fulltext-article-JAA.
Naumov, D., D. Gassan, O. Kotova, E. Sheludko, E. Afanaseva, J. Perelman, et al. 2019. Role of interferon-gamma as a marker of asthma severity and control. European Respiratory Journal [Internet] 54: PA4378. Available from http://erj.ersjournals.com/lookup/doi/10.1183/13993003.congress-2019.PA4378.
Dolhnikoff, M., L.F.F. da Silva, B.B. de Araujo, H.A.P. Gomes, S. Fernezlian, A. Mulder, et al. 2009. The outer wall of small airways is a major site of remodeling in fatal asthma. Journal of Allergy and Clinical Immunology [Internet] 123: 1090–1097.e1. Available from https://linkinghub.elsevier.com/retrieve/pii/S0091674909003832.
Thomson, N.C., R. Chaudhuri, C.M. Messow, M. Spears, W. MacNee, M. Connell, et al. 2013. Chronic cough and sputum production are associated with worse clinical outcomes in stable asthma. Respiratory Medicine [Internet] 107: 1501–1508. Available from https://linkinghub.elsevier.com/retrieve/pii/S0954611113002709.
Woodrow, J.S., M.K. Sheats, B. Cooper, and R. Bayless. 2023. Asthma: the use of animal models and their translational utility. Cells [Internet] 12: 1091. Available from http://www.ncbi.nlm.nih.gov/pubmed/37048164.
Wenzel, S.E., L.B. Schwartz, E.L. Langmack, J.L. Halliday, J.B. Trudeau, R.L. Gibbs, et al. 1999. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. American Journal of Respiratory and Critical Care Medicine [Internet] 160: 1001–1008. Available from https://www.atsjournals.org/doi/10.1164/ajrccm.160.3.9812110.
Theofani, E., M. Semitekolou, I. Morianos, K. Samitas, and G. Xanthou. 2019. Targeting NLRP3 inflammasome activation in severe asthma. Journal of Clinical Medicine [Internet] 8: 1615. Available from https://www.mdpi.com/2077-0383/8/10/1615.
Simpson, J.L., S. Phipps, K.J. Baines, K.M. Oreo, L. Gunawardhana, and P.G. Gibson. 2014. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. European Respiratory Journal [Internet] 43: 1067–1076. Available from http://erj.ersjournals.com/cgi/doi/10.1183/09031936.00105013.
Chen, L., W. Hou, F. Liu, R. Zhu, A. Lv, W. Quan, et al. 2022. Blockade of NLRP3/caspase-1/IL-1β regulated Th17/Treg immune imbalance and attenuated the neutrophilic airway inflammation in an ovalbumin-induced murine model of asthma. Journal of Immunology Research [Internet] 2022: 1–11. Available from https://www.hindawi.com/journals/jir/2022/9444227/.
Schroder, K., and J. Tschopp. 2010. The inflammasomes. Cell [Internet] 140: 821–832. Available from https://linkinghub.elsevier.com/retrieve/pii/S0092867410000759.
Eisenbarth, S.C., O.R. Colegio, W. O’Connor, F.S. Sutterwala, and R.A. Flavell. 2008. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature [Internet] 453: 1122–1126. Available from https://www.nature.com/articles/nature06939.
Chen, M., K. Lin, and S. Lin. 2018. NLRP3 inflammasome signaling as an early molecular response is negatively controlled by miR-186 in CFA-induced prosopalgia mice. Brazilian Journal of Medical and Biological Research [Internet] 51: e7602. Available from http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-879X2018000900607&tlng=en.
Kim, R.Y., J.W. Pinkerton, A.T. Essilfie, A.A.B. Robertson, K.J. Baines, A.C. Brown, et al. 2017. Role for NLRP3 inflammasome–mediated, IL-1β–dependent responses in severe, steroid-resistant asthma. American Journal of Respiratory and Critical Care Medicine [Internet] 196: 283–297. Available from https://www.atsjournals.org/doi/10.1164/rccm.201609-1830OC.
Ma, M., G. Li, M. Qi, W. Jiang, and R. Zhou. 2021. Inhibition of the inflammasome activity of NLRP3 attenuates HDM-induced allergic asthma. Frontiers in Immunology [Internet] 12. Available from https://www.frontiersin.org/articles/10.3389/fimmu.2021.718779/full.
Feng, Y., Y. Wang, P. Wang, Y. Huang, and F. Wang. 2018. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cellular Physiology and Biochemistry [Internet] 49 (1): 190-205. Available from https://doi.org/10.1159/000492853.
Corrêa-Oliveira, R., J.L. Fachi, A. Vieira, F.T. Sato, and M.A.R. Vinolo. 2016. Regulation of immune cell function by short-chain fatty acids. Clinical and Translational Immunology [Internet] 5: e73. Available from http://doi.wiley.com/10.1038/cti.2016.17.
Trompette, A., E.S. Gollwitzer, K. Yadava, A.K. Sichelstiel, N. Sprenger, C. Ngom-Bru, et al. 2014. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nature Medicine [Internet] 20: 159–166. Available from https://www.nature.com/articles/nm.3444.
Maslowski, K.M., A.T. Vieira, A. Ng, J. Kranich, F. Sierro, Yu. Di, et al. 2009. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature [Internet] 461: 1282–1286. Available from https://www.nature.com/articles/nature08530.
Jatakanon, A., C. Uasuf, W. Maziak, S. Lim, K.F. Chung, and P.J. Barnes. 1999. Neutrophilic inflammation in severe persistent asthma. American Journal of Respiratory and Critical Care Medicine [Internet] 160: 1532–1539. Available from https://www.atsjournals.org/doi/10.1164/ajrccm.160.5.9806170.
Howarth, P.H. 2005. Tumour necrosis factor (TNF) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax [Internet] 60: 1012–1018. Available from https://thorax.bmj.com/lookup/doi/10.1136/thx.2005.045260.
Raundhal, M., C. Morse, A. Khare, T.B. Oriss, J. Milosevic, J. Trudeau, et al. 2015. High IFN-γ and low SLPI mark severe asthma in mice and humans. Journal of Clinical Investigation [Internet] 125: 3037–3050. Available from http://www.jci.org/articles/view/80911.
Al-Ramli, W., D. Préfontaine, F. Chouiali, J.G. Martin, R. Olivenstein, C. Lemière, et al. 2009. TH17-associated cytokines (IL-17A and IL-17F) in severe asthma. Journal of Allergy and Clinical Immunology [Internet] 123: 1185–1187. Available from https://linkinghub.elsevier.com/retrieve/pii/S0091674909003479.
Jeffery, P.K. 2004. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society [Internet] 1: 176–183. Available from http://pats.atsjournals.org/cgi/doi/10.1513/pats.200402-009MS.
Fehrenbach, H., C. Wagner, and M. Wegmann. 2017. Airway remodeling in asthma: what really matters. Cell and Tissue Research [Internet] 367: 551–569. Available from http://link.springer.com/10.1007/s00441-016-2566-8.
Chakir, J., J. Shannon, S. Molet, M. Fukakusa, J. Elias, M. Laviolette, et al. 2003. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-β, IL-11, IL-17, and type I and type III collagen expression. Journal of Allergy and Clinical Immunology [Internet] 111: 1293–1298. Available from https://linkinghub.elsevier.com/retrieve/pii/S0091674903013721.
Alagha, K., A. Bourdin, C. Vernisse, C. Garulli, C. Tummino, J. Charriot, et al. 2019. Goblet cell hyperplasia as a feature of neutrophilic asthma. Clinical & Experimental Allergy [Internet] 49: 781–788. Available from https://onlinelibrary.wiley.com/doi/10.1111/cea.13359.
Vieira, S., H. Lemos, R. Grespan, M. Napimoga, D. Dal-Secco, A. Freitas, et al. 2009. A crucial role for TNF-α in mediating neutrophil influx induced by endogenously generated or exogenous chemokines, KC/CXCL1 and LIX/CXCL5. British Journal of Pharmacology [Internet] 158: 779–789. Available from https://onlinelibrary.wiley.com/doi/10.1111/j.1476-5381.2009.00367.x.
Cayrol, C., and J.-P. Girard. 2022. Interleukin-33 (IL-33): a critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine [Internet] 156: 155891. Available from https://linkinghub.elsevier.com/retrieve/pii/S1043466622001004.
Li, Y., W. Wang, Z. Lv, Y. Li, Y. Chen, K. Huang, et al. 2018. Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. The Journal of Immunology [Internet] 200: 2253–2262. Available from https://journals.aai.org/jimmunol/article/200/7/2253/106624/Elevated-Expression-of-IL-33-and-TSLP-in-the.
Gordon, E.M., X. Yao, H. Xu, W. Karkowsky, M. Kaler, O. Kalchiem-Dekel, et al. 2019. Apolipoprotein E is a concentration-dependent pulmonary danger signal that activates the NLRP3 inflammasome and IL-1β secretion by bronchoalveolar fluid macrophages from asthmatic subjects. Journal of Allergy and Clinical Immunology [Internet] 144: 426–441.e3. Available from https://linkinghub.elsevier.com/retrieve/pii/S009167491930346X.
Halwani, R., S. Al-Muhsen, H. Al-Jahdali, and Q. Hamid. 2011. Role of transforming growth factor–β in airway remodeling in asthma. American Journal of Respiratory Cell and Molecular Biology [Internet] 44: 127–133. Available from http://www.atsjournals.org/doi/abs/10.1165/rcmb.2010-0027TR.
Prieto, J., C. Lensmar, A. Roquet, I. Van Der Ploeg, D. Gigliotti, A. Eklund, et al. 2000. Increased interleukin-13 mRNA expression in bronchoalveolar lavage cells of atopic patients with mild asthma after repeated low-dose allergen provocations. Respiratory Medicine [Internet] 94 (8): 806-814. Available from https://doi.org/10.1053/rmed.2000.0826.
Draijer, C., C.E. Boorsma, P. Robbe, W. Timens, M.N. Hylkema, N.H. Ten Hacken, et al. 2017. Human asthma is characterized by more IRF5+ M1 and CD206+ M2 macrophages and less IL-10+ M2-like macrophages around airways compared with healthy airways. Journal of Allergy and Clinical Immunology [Internet] 140: 280–283.e3. Available from http://www.ncbi.nlm.nih.gov/pubmed/28007476.
Khalil, N., O. Bereznay, M. Sporn, and A.H. Greenberg. 1989. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. Journal of Experimental Medicine [Internet] 170: 727–737. Available from https://rupress.org/jem/article/170/3/727/50065/Macrophage-production-of-transforming-growth.
Sun, Q., Y. Wu, F. Zhao, and J. Wang. 2017. Maresin 1 inhibits transforming growth factor-β1-induced proliferation, migration and differentiation in human lung fibroblasts. Molecular Medicine Reports 16 (2): 1523–1529. Available from https://doi.org/10.3892/mmr.2017.6711.
Maltby, S., H.L. Tay, M. Yang, and P.S. Foster. 2017. Mouse models of severe asthma: understanding the mechanisms of steroid resistance, tissue remodelling and disease exacerbation. Respirology [Internet] 22: 874–885. Available from https://onlinelibrary.wiley.com/doi/10.1111/resp.13052.
Carroll, O.R., A.L. Pillar, A.C. Brown, M. Feng, H. Chen, and C. Donovan. 2023. Advances in respiratory physiology in mouse models of experimental asthma. Frontiers in Physiology [Internet] 14: 1099719. Available from https://www.frontiersin.org/articles/10.3389/fphys.2023.1099719/full.
Eisener-Dorman, A.F., D.A. Lawrence, and V.J. Bolivar. 2009. Cautionary insights on knockout mouse studies: the gene or not the gene? Brain, Behavior, and Immunity [Internet] 23: 318–324. Available from https://linkinghub.elsevier.com/retrieve/pii/S0889159108003462.
Franchi, L., and G. Núñez. 2008. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1β secretion but dispensable for adjuvant activity. European Journal of Immunology [Internet] 38: 2085–2089. Available from https://onlinelibrary.wiley.com/doi/10.1002/eji.200838549.
Abdul-Sater, A.A., N. Saïd-Sadier, E.V. Padilla, and D.M. Ojcius. 2010. Chlamydial infection of monocytes stimulates IL-1β secretion through activation of the NLRP3 inflammasome. Microbes and Infectection [Internet] 12: 652–661. Available from https://linkinghub.elsevier.com/retrieve/pii/S128645791000105X.
Rotta detto Loria J, Rohmann K, Droemann D, Kujath P, Rupp J, Goldmann T, et al. 2013. Nontypeable Haemophilus influenzae infection upregulates the NLRP3 inflammasome and leads to caspase-1-dependent secretion of interleukin-1β — a possible pathway of exacerbations in COPD. PLoS One [Internet] 8: e66818. Available from https://dx.plos.org/10.1371/journal.pone.0066818.
Jones, J.T., D.D. Tassew, L.K. Herrera, S.R. Walton-Filipczak, M.A. Montera, H.S. Chand, et al. 2017. Extent of allergic inflammation depends on intermittent versus continuous sensitization to house dust mite. Inhalation Toxicology [Internet] 29: 106–112. Available from https://www.tandfonline.com/doi/full/10.1080/08958378.2017.1311389.
Kim, D.I., M.-K. Song, and K. Lee. 2019. Comparison of asthma phenotypes in OVA-induced mice challenged via inhaled and intranasal routes. BMC Pulmonary Medicine [Internet] 19: 241. Available from https://bmcpulmmed.biomedcentral.com/articles/10.1186/s12890-019-1001-9.
Duan, H., L. Wang, M. Huangfu, and H. Li. 2023. The impact of microbiota-derived short-chain fatty acids on macrophage activities in disease: mechanisms and therapeutic potentials. Biomedicine & Pharmacotherapy [Internet] 165: 115276. Available from https://linkinghub.elsevier.com/retrieve/pii/S0753332223010673.
Arrieta, M.-C., L.T. Stiemsma, P.A. Dimitriu, L. Thorson, S. Russell, S. Yurist-Doutsch, et al. 2015. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Science Translational Medicine [Internet] 7: 152. Available from https://www.science.org/doi/10.1126/scitranslmed.aab2271.
Wang, Z., Z. Lai, X. Zhang, P. Huang, J. Xie, Q. Jiang, et al. 2021. Altered gut microbiome compositions are associated with the severity of asthma. Journal of Thoracic Disease [Internet] 13: 4322–4338. Available from https://jtd.amegroups.com/article/view/54245/html.
Taylor, S.L., L.E.X. Leong, J.M. Choo, S. Wesselingh, I.A. Yang, J.W. Upham, et al. 2018. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. Journal of Allergy and Clinical Immunology [Internet] 141: 94–103.e15 Available from https://linkinghub.elsevier.com/retrieve/pii/S0091674917307431.
Williams, L.M., H.A. Scott, and L.G. Wood. 2019. Soluble fibre as a treatment for inflammation in asthma. Journal of Nutrition and Intermediary Metabolism [Internet] 18: 100108. Available from https://linkinghub.elsevier.com/retrieve/pii/S2352385919300246.
Pouteau, E., I. Meirim, S. Métairon, and L. Fay. 2001. Acetate, propionate and butyrate in plasma: determination of the concentration and isotopic enrichment by gas chromatography/mass spectrometry with positive chemical ionization. Journal of Mass Spectrometry [Internet] 36: 798–805. Available from https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/jms.181.
Thorburn, A.N., C.I. McKenzie, S. Shen, D. Stanley, L. Macia, L.J. Mason, et al. 2015. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nature Communication [Internet] 6: 7320. Available from https://www.nature.com/articles/ncomms8320.
Piyadasa, H., A. Altieri, S. Basu, J. Schwartz, A.J. Halayko, and N. Mookherjee. 2016. Biosignature for airway inflammation in a house dust mite-challenged murine model of allergic asthma. Biology Open [Internet] 5: 112–121. Available from https://journals.biologists.com/bio/article/5/2/112/646/Biosignature-for-airway-inflammation-in-a-house.
Tominaga, K., T. Yoshimoto, K. Torigoe, M. Kurimoto, K. Matsui, T. Hada, et al. 2000. IL-12 synergizes with IL-18 or IL-1β for IFN-γ production from human T cells. International Immunology [Internet] 12: 151–160. Available from https://academic.oup.com/intimm/article-lookup/doi/10.1093/intimm/12.2.151.
De Filippo, K., R.B. Henderson, M. Laschinger, and N. Hogg. 2008. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. The Journal of Immunology [Internet] 180: 4308–4315. Available from https://journals.aai.org/jimmunol/article/180/6/4308/75987/Neutrophil-Chemokines-KC-and-Macrophage.
Kim, J.Y., J.H. Sohn, J.-M. Choi, J.-H. Lee, C.-S. Hong, J.-S. Lee, et al. 2012. Alveolar macrophages play a key role in cockroach-induced allergic inflammation via TNF-α pathway. PLoS One [Internet] 7: e47971. Available from https://dx.plos.org/10.1371/journal.pone.0047971.
Draijer, C., P. Robbe, C.E. Boorsma, M.N. Hylkema, and B.N. Melgert. 2018. Dual role of YM1+ M2 macrophages in allergic lung inflammation. Scientific Reports [Internet] 8: 5105. Available from https://www.nature.com/articles/s41598-018-23269-7.
van der Veen, T.A., L.E.S. de Groot, and B.N. Melgert. 2020. The different faces of the macrophage in asthma. Current Opinion in Pulmonary Medicine [Internet] 26: 62–68. Available from https://journals.lww.com/10.1097/MCP.0000000000000647.
Girodet, P.-O., D. Nguyen, J.D. Mancini, M. Hundal, X. Zhou, E. Israel, et al. 2016. Alternative macrophage activation is increased in asthma. American Journal of Respiratory Cell and Molecular Biology [Internet] 55: 467–475. Available from http://www.atsjournals.org/doi/10.1165/rcmb.2015-0295OC.
Hou, F., K. Xiao, L. Tang, and L. Xie. 2021. Diversity of macrophages in lung homeostasis and diseases. Frontiers in Immunology [Internet] 12: 753940. Available from https://www.frontiersin.org/articles/10.3389/fimmu.2021.753940/full.
Zasłona, Z., S. Przybranowski, C. Wilke, N. van Rooijen, S. Teitz-Tennenbaum, J.J. Osterholzer, et al. 2014. Resident alveolar macrophages suppress, whereas recruited monocytes promote, allergic lung inflammation in murine models of asthma. The Journal of Immunology [Internet] 193: 4245–4253. Available from http://www.jimmunol.org/lookup/doi/10.4049/jimmunol.1400580.
ACKNOWLEDGEMENTS
We acknowledge the generous support of the Science and Technology Institute (ICT) and Center for Development of Experimental Models for Biology and Medicine (CEDEME), both from UNIFESP.
Funding
The study was supported by Coordination for the Improvement of Higher Education Personnel (CAPES) and São Paulo State Research Support Foundation (FAPESP; Grant 2017/19029-2).
Author information
Authors and Affiliations
Contributions
B.S.S.T. contributed to conceptualization, methodology, investigation, and to the writing of the manuscript and review process. F.A. contributed to conceptualization, investigation, and resources. B.S.S.T., M.B.G., R.P.V., A.C.K., K.R.B. contributed to the methodology.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All animal experiments were previously analyzed and approved by the Ethics Committee on the Use of Animal from the Federal University of São Paulo (CEUA—UNIFESP), which follows the National Council for the Control of Animal Experimentation (CONCEA), under the approval number: 5866211118. No human research was conducted.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tagé, B.S.S., Gonzatti, M.B., Vieira, R.P. et al. Three Main SCFAs Mitigate Lung Inflammation and Tissue Remodeling Nlrp3-Dependent in Murine HDM-Induced Neutrophilic Asthma. Inflammation (2024). https://doi.org/10.1007/s10753-024-01983-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-024-01983-x