Abstract
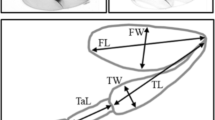
Metric (e.g., body size) and meristic (e.g., bristle number) traits are of general use in quantitative genetic studies, and the phenotypic variance is subdivided into a genetic and a non-genetic environmental component. The non-genetic variance may have two origins: a common garden effect between individuals and a developmental instability within the same individual. Developmental instability may be studied by considering the fluctuating asymmetry (FA) between the two sides of the body. The isofemale line technique is a convenient method for investigating the architecture of natural populations but has been rarely implemented for investigating FA. In this paper, we use this experimental design for analyzing four meristic traits in eight populations of the cosmopolitan Zaprionus indianus. A study of the correlation between left and right side of each line revealed that almost 90% of the variability was due to a developmental noise, while a much higher correlation among the means of the lines from the same population was observed. A slight trend toward a directional asymmetry was observed: more thoracic bristles on the left side. Four kinds of indices, scaled or non-scaled to the mean were used for comparing the different traits. Unscaled values (mean absolute values or standard deviation of each line) revealed a linear increase with the means. Interestingly the results of ovariole number were included in the same regression. With the scaled indices (mean absolute divided by each individual value or stadard deviation devided by the mean), the differences among traits were considerably decreased, but still remained significant. The mean FA of the various traits were not correlated, suggesting that each trait harbors its own developmental stability. The CVs of FA were high with a magnitude similar to those of the trait themselves, slightly less than 10%. Finally, even with the isofemale line design, which is a powerful means for unravelling slight genetic variations, we did not to find any clear indication of a genetic component of FA under the optimal environmental conditions used in this study.



Similar content being viewed by others
References
Allemand R, David JR, Fouillet P (1997) La symétrie bilatérale du nombre de soies ternopleurales: différence entre deux espèces jumelles de Zaprionus (Diptera, Drosophilidae). Arch Zool Expérimentale et Générale 118:123–132
Araripe LO, Klaczko LB, Moreteau B, David JR (2004) Male sterility thresholds in a tropical cosmopolitan drosophilid, Zaprionus indianus. J Therm Biol 29:73–80
Bourguet D (2000) Fluctuating asymmetry and fitness in Drosophila melanogaster. J Evol Biol 13:515–521
Brown NA, Wolpert L (1990) The development of handedness in left/right asymmetry. Development 109:1–9
Carter AJR, Weier TM, Houle D (2009) The effect of inbreeding on fluctuating asymmetry of wing veins in two laboratory strains of Drosophila melanogaster. Heredity 102:563–572
Chakir M, Moreteau B, Capy P, David JR (2007) Phenotypic variability of wild living and laboratory grown Drosophila: consequences of nutritional and thermal heterogeneity in growth conditions. J Therm Biol 32:1–11
Coyne JA, Beecham E (1987) Heritability of two morphological characters within and among natural populations of Drosophila melanogaster. Genetics 117:727–737
David JR, Gilbert P, Legout H, Capy P, Moreteau B (2005) Isofemale lines in Drosophila: an empirical approach to quantitative traits analysis in natural populations. Heredity 94:3–12
David JR, Legout H, Moreteau B (2006a) Phenotypic plasticity of body size in a temperate population of Drosophila melanogaster: when the temperature-size rule does not apply. J Genet 85:9–23
David JR, Araripe LO, Bitner-Mathe BC, Capy P, Goni B, Klaczko LB, Legout H, Martins MB, Vouidibio J, Yassin A, Moreteau B (2006b) Quantitative trait analysis and geographic variability of natural populations of Zaprionus indianus, a recent invader in Brazil. Heredity 96:53–62
David JR, Araripe L, Bitner-Mathe BC, Goni B, Klaczko LB, Legout H, Martins MB, Vouidibio J, Yassin A, Moreteau B (2006c) Sexual dimorphism of body size and sternopleural bristle number: a comparison of geographic populations in an invasive cosmopolitan drosophilid. Genetica 128:109–122
Debat V, Milton CC, Rutherford S, Klingenberg CP, Hoffmann AA (2006) Hsp90 and the quantitative variation of wing shape in Drosophila melanogaster. Evolut Int J org Evolut 60:2529–2538
Debat V, Cornette R, Korol AB, Nevo E, Soulet D, David JR (2008) Multidimensional analysis of Drosophila wing variation in evolution canyon. J Genet 87:407–419
Debat V, Debelle A, Dworkin I (2009) Plasticity, canalization, and developmental stability of the Drosophila wing: joint effects of mutations and developmental temperature. Evolut Int J org Evolut 63–11:2864–2876
Debat V, Bloyer S, Faradji F, Gidaszewski N, Navarro N, Orozco-Terwengel P, et al (2011) Developmental stability: a major role for Cyclin G in Drosophila melanogaster. PLoS Genet 7:e1002314 (PMID: 21998598)
Delpuech JM, Moreteau B, Chiche J, Pla E, Vouidibio J, David JR (1995) Phenotypic plasticity and reaction norms in temperate and tropical populations of Drosophila melanogaster: ovarian size and developmental temperature. Evolut Int J Org Evolut 49(4):670–676
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, edn 4. Longmans Green, Harlow
Fowler K, Whitlock MC (1994) Fluctuating asymmetry does not increase with moderate inbreeding in Drosophila melanogaster. Heredity 73:373–376
Gibert P, Moreteau B, Moreteau JC et al (1998) Genetic variability in quantitative traits in Drosophila Melanogaster (fruit fly) natural populations: analysis of wild-living flies and of several laboratory generations. Heredity 80:326–335
Giddings LV, Kaneshiro KY, Anderson W (1989) Genetics, speciation and the founder principle. Oxford Univ. Press, Oxford
Gómez FH, Norry FM (2012) Is the number of possible QTL for asymmetry phenotypes dependent on thermal stress? J Therm Biol 37(1):1–5
Graham JH, Freeman DC, Emlen JM (1993) Antisymmetry, directional asymmetry, and dynamic morphogenesis. Genetica 89:121–137
Imasheva AG, Loeschcke V, Zhivotovsky DA, Lazebny OE (1997) Effects of extreme temperatures on phenotypic variation and developmental stability in Drosophila melanogaster and Drosophila buzzatii. Biol J Linn Soc 61:117–126
Imasheva AG, Bosenko DV, Bubli OA (1999) Variation in morphological traits of Drosophila melanogaster (fruit fly) under nutritional stress. Heredity 82:187–192
Indrasamy H, Woods RE, McKenzie JA, Batterham P (2000) Fluctuating asymmetry for specific bristle characters in Notch mutants of Drosophila melanogaster. Genetica 109:151–159
Karan D, Morin JP, Gravot E, Moreteau B, David JR (1999) Body size reaction norms in Drosophila melanogaster: temporal stability and genetic architecture in a natural population. Genet Sel Evol 31:491–508
Klingenberg CP, Zaklan SD (2000) Morphological integration between developmental compartments in the Drosophila wing. Evolut Int J Org Evolut 54:1273–1275
Kurbalija Z, Stamenkovic-Radak M, Pertoldi C, Andjelkovic M (2010) Outbreeding causes developmental instability in Drosophila subobscura. Evolut Ecol 24(4):839–864
Levin M, Palmer AR (2007) Left–right patterning from the inside out: widespread evidence for intracellular control. Bioessays 29:271–287
Lynch M, Walsh B (1998) Genetics and analysis of quantitative traits. Sinauer, Sunderland
Markow TA, Ricker IP (1992) Male size developmental stability, and mating success in natural populations of three Drosophila species. Heredity 69:122–127
Maynard Smith J, Szathmary E (1995) The major transitions in evolution. Oxford University Press, Oxford
McManus IC (2002) Right hand, left hand: the origins of asymmetry in brains, bodies, atoms and cultures. Weidenfeld and Nicolson/Harvard University Press, London
Møller AP, Swadle JP (1997) Asymmetry, developmental stability and evolution. Oxford Univ Press, Oxford
Moreteau B, Brochen A, Pla E, David JR (2000) Ovarian fluctuating asymmetry: a stable property among Drosophila species. Drosoph Inf Ser 83:51–54
Nakamura, T, Hamada, H (2012) Left–right patterning: conserved and divergent mechanisms. Development 139:3257–3262
Palmer AR (1994) Fluctuating asymmetry analyses: a primer. In: Markow TA (ed) Developmental instability: its origin and evolutionary implications. Kluwer, Dordrecht, pp 335–364
Palmer AR (1996) Waltzing with asymmetry. Bioscience 46:518–536
Palmer AR (1999) Detecting publication bias in Meta-analyses: a case study of fluctuating asymmetry and sexual selection. Am Nat 154:220–233
Palmer AR, Strobeck C (1986) Fluctuating asymmetry: measurement, analysis, patterns. Ann Rev Ecol Syst 17:391–421
Palmer AR, Strobeck C (1992) Fluctuating asymmetry as a measure of developmental stability: implications of non-normal distributions and power of statistical tests. Acta Zool Fenn 191:55–70
Palmer AR, Strobeck C (2003) Fluctuating asymmetry analysis revisited. In: Polak M (ed). Oxford University Press, Oxford, pp 279–319
Pétavy G, Morin JP, Moreteau B, David JR (1997) Growth temperature and phenotypic plasticity in two Drosophila sibling species: probable adaptive changes in flight capacities. J Evol Biol 10:875–887
Pétavy G, David JR, Debat V, Pertoldi C, Moreteau B (2006) Phenotypic and genetic variability of sternopleural bristle number in Drosophila melanogaster under daily thermal stress: developmental instability and anti-asymmetry. Evol Ecol Res 8:149–167
Polak M (2003) Developmental instability: causes and consequences. Oxford University Press, Oxford
Roff DA, Mousseau TA (1987) Quantitative genetics and fitness: lessons from Drosophila. Heredity 58:103–118
Santos M (2001) Fluctuating asymmetry is non-genetically related to mating success in Drosophila buzzatii. Evolut Int J Org Evolut 55:2248–2256
Simpson P, Woehl R, Usui K (1999) The development and evolution of bristle patterns in Diptera. Development 126(7):1349–1364
Soto IM, Carreira VP, Soto EM, Hasson E (2008) Wing morphology and fluctuating asymmetry depend on the host plant in catophilic Drosophila. J Evol Biol 21:598–609
Swain DF (1987) A problem with the use of meristic characters to estimate developmental stability. Am Nat 129:761–768
Trotta V, Calboli, FCF, Garoia F, Grifoni D, Cavicchi S (2005) Fluctuating asymmetry as measure of ecological stress in Drosophila melanogaster (Diptera: Drosophilidae). Eur J Entomol 102:195–200
Vandenberg LN, Levin M (2013) A unified model for left–right asymmetry? Comparison and synthesis of molecular models of embryonic laterality. Dev Biol 379:1–15
Vilela CR (1999) Is Zaprionus indianus Gupta, 1970 (Diptera, Drosophilidae) currently colonizing the neotropical region? Drosoph Inf Ser 82:37–39
Vishalakshi C, Singh BN (2008) Effects of developmental temperature stress on fluctuating asymmetry in certain morphological traits in Drosophila ananassae. J Therm Biol 33:201–208
Waddington CH (1957) The strategy of the genes. George Allen Unwin London, UK
Whitlock, M (1996) The heritability of fluctuating asymmetry and the genetic control of developmental stability. Proc R Soc Lond B Biol 263(1372):849–853
Woods RE, Hercus MJ, Hoffmann AA (1998) Estimating the heritability of fluctuating asymmetry in field Drosophila. Evolut Int J org Evolut 52:816–824
Woods RE, Sgro CM, Hercus MJ, Hoffmann AA (1999) The association between fluctuating asymmetry, trait variability, trait heritability, and stress: a multiply replicated experiment on combined stresses in Drosophila melanogaster. Evolution 53:493–505
Woods RE, Sgro CM, Hercus MJ, Hoffmann AA (2002) Fluctuating asymmetry, fecundity and development time in Drosophila: is there an association under optimal and stress conditions? J Evol Biol 15:146–157
Yassin A, David JR, Bitner-Mathé BC (2009) Phenotypic variability of natural populations of an invasive drosophilid, Zaprionus indianus, on different continents: comparison of wild-living and laboratory-grown flies. C R Biol 332:898–908
Acknowledgements
We thank Blanche Bittner Mathé for comments and suggestions on data in this paper. This work was supported by Coordenação de Aperfeiçoamento de Nível Superior (CAPES) and Comite Français d’Evaluation de la Coopéation Universitaire avec le Brésil (COFECUBE). This paper results from a French-Brazilian cooperation programme on Zaprionus.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madi-Ravazzi, L., Segala, L.F., Debat, V. et al. Fluctuating asymmetry of meristic traits: an isofemale line analysis in an invasive drosophilid, Zaprionus indianus . Genetica 145, 307–317 (2017). https://doi.org/10.1007/s10709-017-9966-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-017-9966-x