Abstract
Background
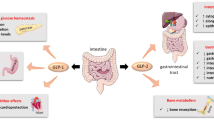
Therapy with nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with enteropathy in humans and experimental animals, a cause of considerable morbidity. Unlike foregut NSAID-associated mucosal lesions, most treatments for this condition are of little efficacy. We propose that the endogenously released intestinotrophic hormone glucagon-like peptide-2 (GLP-2) prevents the development of NSAID-induced enteropathy. Since the short-chain fatty acid receptor FFA3 is expressed on enteroendocrine L cells and on enteric nerves in the gastrointestinal tract, we further hypothesized that activation of FFA3 on L cells protects the mucosa from injury via GLP-2 release with enhanced duodenal HCO3 − secretion. We thus investigated the effects of synthetic selective FFA3 agonists with consequent GLP-2 release on NSAID-induced enteropathy.
Methods
We measured duodenal HCO3 − secretion in isoflurane-anesthetized rats in a duodenal loop perfused with the selective FFA3 agonists MQC or AR420626 (AR) while measuring released GLP-2 in the portal vein (PV). Intestinal injury was produced by indomethacin (IND, 10 mg/kg, sc) with or without MQC (1–10 mg/kg, ig) or AR (0.01–0.1 mg/kg, ig or ip) treatment.
Results
Luminal perfusion with MQC or AR (0.1–10 µM) dose-dependently augmented duodenal HCO3 − secretion accompanied by increased GLP-2 concentrations in the PV. The effect of FFA3 agonists was inhibited by co-perfusion of the selective FFA3 antagonist CF3-MQC (30 µM). AR-induced augmented HCO3 − secretion was reduced by iv injection of the GLP-2 receptor antagonist GLP-2(3-33) (3 nmol/kg), or by pretreatment with the cystic fibrosis transmembrane conductance regulator (CFTR) inhibitor CFTRinh-172 (1 mg/kg, ip). IND-induced small intestinal ulcers were dose-dependently inhibited by intragastric administration of MQC or AR. GLP-2(3-33) (1 mg/kg, ip) or CF3-MQC (1 mg/kg, ig) reversed AR-associated reduction in IND-induced enteropathy. In contrast, ip injection of AR had no effect on enteropathy.
Conclusion
These results suggest that luminal FFA3 activation enhances mucosal defenses and prevents NSAID-induced enteropathy via the GLP-2 pathway. The selective FFA3 agonist may be a potential therapeutic candidate for NSAID-induced enteropathy.





Similar content being viewed by others
Abbreviations
- 5-HT:
-
5-Hydroxytryptamine
- CF3-MQC:
-
N-(2-methylphenyl)-4-[5-(2-trifluoromethoxy-phenyl)-furan-2-yl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxamide
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- DPP4:
-
Dipeptidyl peptidase 4
- FFA3:
-
Free fatty acid receptor 3
- GPCR:
-
G protein-coupled receptor
- GLP:
-
Glucagon-like peptide
- IND:
-
Indomethacin
- MQC:
-
N-[2-methylphenyl]-[4-furan-3-yl]-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxamide
- PV:
-
Portal vein
- SCFA:
-
Short-chain fatty acid
References
Higuchi K, Umegaki E, Watanabe T, et al. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009;44:879–888.
Matsumoto T, Kudo T, Esaki M, et al. Prevalence of non-steroidal anti-inflammatory drug-induced enteropathy determined by double-balloon endoscopy: a Japanese multicenter study. Scand J Gastroenterol. 2008;43:490–496.
Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55–59.
Wallace JL. NSAID gastropathy and enteropathy: distinct pathogenesis likely necessitates distinct prevention strategies. Br J Pharmacol. 2012;165:67–74.
Omatsu T, Naito Y, Handa O, et al. Reactive oxygen species-quenching and anti-apoptotic effect of polaprezinc on indomethacin-induced small intestinal epithelial cell injury. J Gastroenterol. 2010;45:692–702.
Duggan DE, Hooke KF, Noll RM, Kwan KC. Enterohepatic circulation of indomethacin and its role in intestinal irritation. Biochem Pharmacol. 1975;24:1749–1754.
Iwai T, Ichikawa T, Kida M, et al. Vulnerable sites and changes in mucin in the rat small intestine after non-steroidal anti-inflammatory drugs administration. Dig Dis Sci. 2010;55:3369–3376.
Shimizu K, Koga H, Iida M, Haruma K. Microcirculatory changes in experimental mesenteric longitudinal ulcers of the small intestine in rats. Dig Dis Sci. 2007;52:3019–3028.
Takeuchi K, Kita K, Hayashi S, Aihara E. Regulatory mechanism of duodenal bicarbonate secretion: roles of endogenous prostaglandins and nitric oxide. Pharmacol Ther. 2011;130:59–70.
Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Dynamic regulation of mucus gel thickness in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2000;279:G437–G447.
Takeuchi K, Miyazawa T, Tanaka A, Kato S, Kunikata T. Pathogenic importance of intestinal hypermotility in NSAID-induced small intestinal damage in rats. Digestion. 2002;66:30–41.
Konaka A, Kato S, Tanaka A, Kunikata T, Korolkiewicz R, Takeuchi K. Roles of enterobacteria, nitric oxide and neutrophil in pathogenesis of indomethacin-induced small intestinal lesions in rats. Pharmacol Res. 1999;40:517–524.
Watanabe T, Higuchi K, Kobata A, et al. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut. 2008;57:181–187.
Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314–1322.
Uchida A, Yamada T, Hayakawa T, Hoshino M. Taurochenodeoxycholic acid ameliorates and ursodeoxycholic acid exacerbates small intestinal inflammation. Am J Physiol. 1997;272:G1249–G1257.
Inoue T, Higashiyama M, Kaji I, et al. Dipeptidyl peptidase IV inhibition prevents the formation and promotes the healing of indomethacin-induced intestinal ulcers in rats. Dig Dis Sci. 2014;59:1286–1295.
Guan X, Karpen HE, Stephens J, et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150–164.
Wang JH, Inoue T, Higashiyama M, et al. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J Pharmacol Exp Ther. 2011;339:464–473.
Boushey RP, Yusta B, Drucker DJ. Glucagon-like peptide 2 decreases mortality and reduces the severity of indomethacin-induced murine enteritis. Am J Physiol. 1999;277:E937–E947.
Rønnestad I, Akiba Y, Kaji I, Kaunitz JD. Duodenal luminal nutrient sensing. Curr Opin Pharmacol. 2014;19C:67–75.
Akiba Y, Inoue T, Kaji I, et al. Short-chain fatty acid sensing in rat duodenum. J Physiol. 2015;593:585–599.
Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky de Muckadell OB, Ainsworth MA. CFTR mediates cAMP- and Ca2+-activated duodenal epithelial HCO3− secretion. Am J Physiol. 1997;272:G872–G878.
Ulven T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front Endocrinol (Lausanne). 2012;3:111.
Akiba Y, Jung M, Ouk S, Kaunitz JD. A novel small molecule CFTR inhibitor attenuates HCO3 − secretion and duodenal ulcer formation in rats. Am J Physiol Gastrointest Liver Physiol. 2005;289:G753–G759.
Kaji I, Iwanaga T, Watanabe M, et al. SCFA transport in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2015;308:G188–G197.
Mizumori M, Meyerowitz J, Takeuchi T, et al. Epithelial carbonic anhydrases facilitate PCO2 and pH regulation in rat duodenal mucosa. J Physiol. 2006;573:827–842.
Kaji I, Akiba Y, Konno K, et al. Neural FFA3 activation inversely regulates anion secretion evoked by nicotinic ACh receptor activation in rat proximal colon. J Physiol. 2016;594:3339–3352.
Kaji I, Akiba Y, Furuyama T, Kaunitz JD. FFA3 activation inhibits nicotine-induced secretion and motility via enteric nervous reflex in rat proximal colon. Gastroenterology. 2016;150:S352.
Inoue T, Wang JH, Higashiyama M, et al. Dipeptidyl peptidase IV inhibition potentiates amino acid- and bile acid-induced bicarbonate secretion in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2012;303:G810–G816.
Akiba Y, Kaunitz JD. Duodenal chemosensing and mucosal defenses. Digestion. 2011;83:25–31.
Hudson BD, Christiansen E, Murdoch H, et al. Complex pharmacology of novel allosteric free fatty acid 3 receptor ligands. Mol Pharmacol. 2014;86:200–210.
Brown AJ, Goldsworthy SM, Barnes AA, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319.
Amelsberg M, Amelsberg A, Ainsworth MA, Hogan DL, Isenberg JI. Cyclic adenosine-3′,5′-monophosphate production is greater in rabbit duodenal crypt than in villus cells. Scand J Gastroenterol. 1996;31:233–239.
Ameen NA, Martensson B, Bourguinon L, Marino C, Isenberg J, McLaughlin GE. CFTR channel insertion to the apical surface in rat duodenal villus epithelial cells is upregulated by VIP in vivo. J Cell Sci. 1999;112:887–894.
Bahrami J, Yusta B, Drucker DJ. ErbB activity links the glucagon-like peptide-2 receptor to refeeding-induced adaptation in the murine small bowel. Gastroenterology. 2010;138:2447–2456.
Dong CX, Zhao W, Solomon C, et al. The intestinal epithelial insulin-like growth factor-1 receptor links glucagon-like peptide-2 action to gut barrier function. Endocrinology. 2014;155:370–379.
Nøhr MK, Egerod KL, Christiansen SH, et al. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience. 2015;290:126–137.
Akiba Y, Kaji I, Guth PH, Engel E, Kaunitz JD. GPR43 activation with COX inhibition induces duodenal injury via 5-HT pathway. Gastroenterology. 2014;146:S504–S505.
Fujiwara K, Inoue T, Yorifuji N, et al. Combined treatment with dipeptidyl peptidase 4 (DPP4) inhibitor sitagliptin and elemental diets reduced indomethacin-induced intestinal injury in rats via the increase of mucosal glucagon-like peptide-2 concentration. J Clin Biochem Nutr. 2015;56:155–162.
Sakanaka T, Inoue T, Yorifuji N, et al. The effects of a TGR5 agonist and a dipeptidyl peptidase IV inhibitor on dextran sulfate sodium-induced colitis in mice. J Gastroenterol Hepatol. 2015;30:60–65.
Acknowledgments
We thank Stacey Jung for her assistance with manuscript preparation and Drs. Paul Guth and Eli Engel for their helpful discussion.
Funding
This work was supported by a Department of Veterans Affairs Merit Review Award and NIH R01 DK54221.
Author information
Authors and Affiliations
Contributions
Y.A. and J.D.K. were responsible for the study concept and design, and for drafting article. Ay.K. and K.I. were responsible for chemical design and synthesis. H.S., Y.A., K.N., and K.M. were responsible for collection, assembly, and analysis of data. Y.A., At.K., and J.D.K. were responsible for data interpretation.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Said, H., Akiba, Y., Narimatsu, K. et al. FFA3 Activation Stimulates Duodenal Bicarbonate Secretion and Prevents NSAID-Induced Enteropathy via the GLP-2 Pathway in Rats. Dig Dis Sci 62, 1944–1952 (2017). https://doi.org/10.1007/s10620-017-4600-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4600-4