Abstract
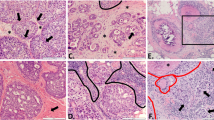
Mortality in breast cancer is linked to metastasis and recurrence yet there is no acceptable biological model for cancer relapse. We hypothesise that there might exist primary tumour cells capable of escaping surgery by migration and resisting radiotherapy and chemotherapy to cause cancer recurrence. We investigated this possibility in invasive ductal carcinoma (IDC) tissue and observed the presence of solitary primary tumour cells (SPCs) in the dense collagen stroma that encapsulates intratumoural cells (ICs). In IDC tissue sections, collagen was detected with either Masson’s Trichrome or by second harmonics imaging. Cytokeratin-19 (CK-19) and vimentin (VIM) antibodies were, respectively, used to identify epithelial-derived tumour cells and to indicate epithelial to mesenchymal transition (EMT). Confocal/multiphoton microscopy showed that ICs from acini were mainly CK-19+ve and were encapsulated by dense stromal collagen. Within the stroma, SPCs were detected by their staining for both CK-19 and VIM (confirming EMT). ICs and SPCs were subsequently isolated by laser capture microdissection followed by multiplex tandem-PCR studies. SPCs were found to be enriched for pro-migratory and anti-proliferative genes relative to ICs. In vitro experiments using collagen matrices at 20 mg/cm3, similar in density to tumour matrices, demonstrated that SPC-like cells were highly migratory but dormant, phenotypes that recapitulated the genotypes of SPCs in clinical tissue. These data suggest that SPCs located at the breast cancer perimeter are invasive and dormant such that they may exceed surgical margins and resist local and adjuvant therapies. This study has important connotations for a role of SPCs in local recurrence.











Similar content being viewed by others
Abbreviations
- BrCa:
-
Breast cancer
- CK:
-
Cytokeratin
- CTCs:
-
Circulating tumour cells
- DCIS:
-
Ductal carcinoma in situ
- DTCs:
-
Disseminated tumour cells
- EMT:
-
Epithelial mesenchymal transition
- FFPE:
-
Formalin-fixed-paraffin-embedded
- ICs:
-
Intratumoural cells
- IDC:
-
Invasive ductal carcinoma
- LCM:
-
Laser capture microdissection
- MT-PCR:
-
Multiplexed tandem-PCR
- SPCs:
-
Solitary primary tumour cells
- VIM:
-
Vimentin
References
Wyckoff JB et al (2000) A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res 60(9):2504–2511
Pantel K et al (2003) Detection and clinical implications of early systemic tumor cell dissemination in breast cancer. Clin Cancer Res 9(17):6326–6334
Braun S et al (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353(8):793–802
Hedley BD, Chambers AF (2009) Tumor dormancy and metastasis. Adv Cancer Res 102:67–101
Li CI, Daling JR (2007) Changes in breast cancer incidence rates in the United States by histologic subtype and race/ethnicity, 1995 to 2004. Cancer Epidemiol Biomarkers Prev 16(12):2773–2780
Sinha PS, Bendall S, Bates T (2000) Does routine grading of invasive lobular cancer of the breast have the same prognostic significance as for ductal cancers? Eur J Surg Oncol 26(8):733–737
Wyckoff J et al (2004) A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 64(19):7022–7029
Wyckoff JB et al (2007) Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res 67(6):2649–2656
Robinson BD et al (2009) Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res 15(7):2433–2441
Gertler F, Condeelis J (2011) Metastasis: tumor cells becoming MENAcing. Trends Cell Biol 21(2):81–90
Meng S et al (2004) Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res 10(24):8152–8162
Aguirre-Ghiso JA, Ossowski L, Rosenbaum SK (2004) Green fluorescent protein tagging of extracellular signal-regulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer Res 64(20):7336–7345
Indraccolo S et al (2006) Interruption of tumor dormancy by a transient angiogenic burst within the tumor microenvironment. Proc Natl Acad Sci USA 103(11):4216–4221
Naumov GN et al (2006) A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J Natl Cancer Inst 98(5):316–325
Woodcock-Mitchell J et al (1982) Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J Cell Biol 95(2 Pt 1):580–588
Folpe AL, Cooper K (2007) Best practices in diagnostic immunohistochemistry: pleomorphic cutaneous spindle cell tumors. Arch Pathol Lab Med 131(10):1517–1524
Stanley KK, Szewczuk E (2005) Multiplexed tandem PCR: gene profiling from small amounts of RNA using SYBR Green detection. Nucleic Acids Res 33(20):e180
Whitehead RH et al (1983) A new human breast carcinoma cell line (PMC42) with stem cell characteristics. I. Morphologic characterization. J Natl Cancer Inst 70(4):649–661
Sakaue-Sawano A et al (2008) Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132(3):487–498
Sapino A, Frigerio A, Peterse JL, Arisio R, Coluccia C, Bussolati G (2000) Mammographically detected in situ lobular carcinomas of the breast. Virchows Arch 436(5):421–430
Lee JM et al (2006) The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 172(7):973–981
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119(6):1420–1428
Mendez MG, Kojima S, Goldman RD (2010) Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J 24(6):1838–1851
Yasui W et al (2004) Search for new biomarkers of gastric cancer through serial analysis of gene expression and its clinical implications. Cancer Sci 95(5):385–392
Fonsato V et al (2006) Expression of Pax2 in human renal tumor-derived endothelial cells sustains apoptosis resistance and angiogenesis. Am J Pathol 168(2):706–713
Chen Q, DeGraff DJ, Sikes RA (2010) The developmental expression profile of PAX2 in the murine prostate. Prostate 70(6):654–665
Chu CY et al (2008) Connective tissue growth factor (CTGF) and cancer progression. J Biomed Sci 15(6):675–685
Barsky SH et al (1997) ‘Revertant’ DCIS in human axillary breast carcinoma metastases. J Pathol 183(2):188–194
Lipponen P et al (1994) Tumour vascularity and basement membrane structure in breast cancer as related to tumour histology and prognosis. J Cancer Res Clin Oncol 120(11):645–650
Pierga JY et al (2004) Clinical significance of immunocytochemical detection of tumor cells using digital microscopy in peripheral blood and bone marrow of breast cancer patients. Clin Cancer Res 10(4):1392–1400
Muller V et al (2005) Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res 11(10):3678–3685
Klein CA (2003) The systemic progression of human cancer: a focus on the individual disseminated cancer cell: the unit of selection. Adv Cancer Res 89:35–67
Husemann Y et al (2008) Systemic spread is an early step in breast cancer. Cancer Cell 13(1):58–68
Thompson EW, Newgreen DF, Tarin D (2005) Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res 65(14):5991–5995 discussion 5
Sorlie T et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98(19):10869–10874
Hollier BG, Evans K, Mani SA (2009) The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia 14(1):29–43
Mani SA et al (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133(4):704–715
Li C, Lee CJ, Simeone DM (2009) Identification of human pancreatic cancer stem cells. Methods Mol Biol 568:161–173
Creighton CJ, Chang JC, Rosen JM (2010) Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neoplasia 15(2):253–260
Armstrong AJ et al (2011) Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res 9(8):997–1007
Blick T et al (2010) Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/-) stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia 15(2):235–252
Trimboli AJ et al (2008) Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res 68(3):937–945
Yoder BJ et al (2005) The expression of fascin, an actin-bundling motility protein, correlates with hormone receptor-negative breast cancer and a more aggressive clinical course. Clin Cancer Res 11(1):186–192
Cooper D, Schermer A, Sun TT (1985) Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest 52(3):243–256
Datta YH et al (1994) Sensitive detection of occult breast cancer by the reverse-transcriptase polymerase chain reaction. J Clin Oncol 12(3):475–482
Braun S et al (2000) Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med 342(8):525–533
Kallergi G et al (2007) Phosphorylation of FAK, PI-3K, and impaired actin organization in CK-positive micrometastatic breast cancer cells. Mol Med 13(1–2):79–88
Becker S et al (2009) Evaluation of a RT-PCR based routine screening tool for the detection of disseminated epithelial cells in the bone marrow of breast cancer patients. Breast Cancer Res Treat 117(2):227–233
Moody SE et al (2005) The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 8(3):197–209
Lang SH et al (2002) Enhanced expression of vimentin in motile prostate cell lines and in poorly differentiated and metastatic prostate carcinoma. Prostate 52(4):253–263
Perou CM et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752
Sorlie T et al (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100(14):8418–8423
Prat A et al (2010) Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 12(5):R68
Wang W et al (2004) Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res 64(23):8585–8594
Barr FG (1997) Chromosomal translocations involving paired box transcription factors in human cancer. Int J Biochem Cell Biol 29(12):1449–1461
Muratovska A et al (2003) Paired-box genes are frequently expressed in cancer and often required for cancer cell survival. Oncogene 22(39):7989–7997
Gnarra JR, Dressler GR (1995) Expression of Pax-2 in human renal cell carcinoma and growth inhibition by antisense oligonucleotides. Cancer Res 55(18):4092–4098
Silberstein GB, Dressler GR, Van Horn K (2002) Expression of the PAX2 oncogene in human breast cancer and its role in progesterone-dependent mammary growth. Oncogene 21(7):1009–1016
Buttiglieri S et al (2004) Role of Pax2 in apoptosis resistance and proinvasive phenotype of Kaposi’s sarcoma cells. J Biol Chem 279(6):4136–4143
Roy-Burman P et al (2004) Genetically defined mouse models that mimic natural aspects of human prostate cancer development. Endocr Relat Cancer 11(2):225–254
Peehl DM (2005) Primary cell cultures as models of prostate cancer development. Endocr Relat Cancer 12(1):19–47
Xiaogang R et al (2008) Study of tumor molecular diagnosis model based on artificial neural network with gene expression profile. Evolutionary Computation, 2008 CEC 2008 (IEEE World Congress on Computational Intelligence) IEEE Congress on 1–6 June 2008, p 1051–1056
Wong AS, Gumbiner BM (2003) Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin. J Cell Biol 161(6):1191–1203
Larue L, Bellacosa A (2005) Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 24(50):7443–7454
Klymkowsky MW, Savagner P (2009) Epithelial-mesenchymal transition: a cancer researcher’s conceptual friend and foe. Am J Pathol 174(5):1588–1593
Jechlinger M et al (2003) Expression profiling of epithelial plasticity in tumor progression. Oncogene 22(46):7155–7169
Barbolina MV et al (2009) Downregulation of connective tissue growth factor by three-dimensional matrix enhances ovarian carcinoma cell invasion. Int J Cancer 125(4):816–825
Brigstock DR (1999) The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev 20(2):189–206
Sahai E (2005) Mechanisms of cancer cell invasion. Curr Opin Genet Dev 15(1):87–96
Zheng HC et al (2008) Arp2/3 overexpression contributed to pathogenesis, growth and invasion of gastric carcinoma. Anticancer Res 28(4B):2225–2232
Rohatgi R, Ho HY, Kirschner MW (2000) Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4,5-bisphosphate. J Cell Biol 150(6):1299–1310
Mizutani K et al (2002) Essential role of neural Wiskott–Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res 62(3):669–674
Yamaguchi H et al (2005) Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol 168(3):441–452
Innocenti M et al (2004) Abi1 is essential for the formation and activation of a WAVE2 signalling complex. Nat Cell Biol 6(4):319–327
Kurisu S et al (2005) Rac-WAVE2 signaling is involved in the invasive and metastatic phenotypes of murine melanoma cells. Oncogene 24(8):1309–1319
Rodriguez-Pinilla SM et al (2006) Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res 12(5):1533–1539
Xing P et al (2011) Fascin, an actin-bundling protein, promotes breast cancer progression in vitro. Cell Biochem Funct 29(4):303–310. doi:10.1002/cbf.1750
Leung T et al (1995) A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem 270(49):29051–29054
Bhadriraju K et al (2007) Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp Cell Res 313(16):3616–3623
Riento K, Ridley AJ (2003) Rocks: multifunctional kinases in cell behaviour. Natl Rev Mol Cell Biol 4(6):446–456
Bourguignon LY et al (1999) Rho-kinase (ROK) promotes CD44v(3,8-10)-ankyrin interaction and tumor cell migration in metastatic breast cancer cells. Cell Motil Cytoskelet 43(4):269–287
Yoshioka K, Nakamori S, Itoh K (1999) Overexpression of small GTP-binding protein RhoA promotes invasion of tumor cells. Cancer Res 59(8):2004–2010
Hunter T, Pines J (1994) Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell 79(4):573–582
Musgrove EA et al (1996) Cyclins and breast cancer. J Mammary Gland Biol Neoplasia 1(2):153–162
Zhang H, Xiong Y, Beach D (1993) Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol Biol Cell 4(9):897–906
Polyak K et al (1994) Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78(1):59–66
Han SH et al (2003) VDUP1 upregulated by TGF-beta1 and 1,25-dihydorxyvitamin D3 inhibits tumor cell growth by blocking cell-cycle progression. Oncogene 22(26):4035–4046
Steeg PS, Zhou Q (1998) Cyclins and breast cancer. Breast Cancer Res Treat 52(1–3):17–28
Christov K et al (2003) Cell proliferation, apoptosis, and expression of cyclin D1 and cyclin E as potential biomarkers in tamoxifen-treated mammary tumors. Breast Cancer Res Treat 77(3):253–264
Sheth SS et al (2005) Thioredoxin-interacting protein deficiency disrupts the fasting-feeding metabolic transition. J Lipid Res 46(1):123–134
Sheth SS et al (2006) Hepatocellular carcinoma in Txnip-deficient mice. Oncogene 25(25):3528–3536
Yang X, Young LH, Voigt JM (1998) Expression of a vitamin D-regulated gene (VDUP-1) in untreated- and MNU-treated rat mammary tissue. Breast Cancer Res Treat 48(1):33–44
Willipinski-Stapelfeldt B et al (2005) Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res 11(22):8006–8014
Raviraj V, Fok S, Zhao J, Chien HY, Lyons JG, Thompson EW, Soon L (2011) Regulation of ROCK1 via Notch1 during breast cancer cell migration into dense matrices. BMC Cell Biology (submitted)
Acknowledgments
This work was supported by National Health and Medical Research Council (#402510 and #571200), Australian Research Council (#DP0881012) and Victorian Breast Cancer Research Consortium. We gratefully acknowledge AMMRF, ACMM, Sydney, Australia for use of the facility and Ms Ellie Kable, Dr Renee Whan, Mr Vivek Ravichandran and Mr Dennis Dwarte for their kind assistance. We thank Prof. John Condeelis for kindly providing the MTLn3 cells and Assoc. Prof. Prue Hill, St. Vincent’s Pathology, Melbourne, for assistance with histopathological assessments of tissues.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raviraj, V., Zhang, H., Chien, Hy. et al. Dormant but migratory tumour cells in desmoplastic stroma of invasive ductal carcinomas. Clin Exp Metastasis 29, 273–292 (2012). https://doi.org/10.1007/s10585-011-9450-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-011-9450-4