Abstract
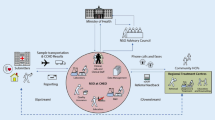
In many European countries neonatal screening has been introduced over the last 50 years as an important public health programme. Depending on health care structure, available funds, local politics, input from professional groups, parent groups, and the general public this introduction has led to different approaches in the way the screening programmes have been set up, financed and governed. To get some insight about the current situation, in 2009 the European Union, via its EAHC agency, put out a call for a tender that was acquired by our project group. An online survey was compiled in which the whole screening programme was covered by a questionnaire. This survey covered the EU member states, (potential) candidate member states and EFTA countries, in total 40 countries. Results showed little consensus concerning 1. information of parents including informed consent; 2. which conditions are screened for, ranging from 1 to around 30 conditions; 3. sampling time post partum; 4. screening methodology including cut-offs values even between screening laboratories within countries.; 5. storage of residual specimens, varying from 3 months to 1000 years. In addition, confirmatory diagnostics and follow-up also show large discrepancies (Burgard et al. http://www.iss.it/cnmr/prog/cont.php?id=1621&lang=1&tipo=64 2011). In addition to the current practices report an expert opinion document has been produced with recommendations to the EU Commission for future improvements, e.g. in parallel to the way the USA has harmonized its practices based on recommendations by the American College of Medical Genetics (Watson et al., Pediatrics 117: S296-S307, 2006).
Similar content being viewed by others
Abbreviations
- 3HMG:
-
3-Hydroxy-3-methylglutaric aciduria
- 3MCC:
-
3-Methylcrotonyl-CoA carboxylase deficiency/3-Methylglutacon aciduria/2-methyl-3-OH-butyric aciduria
- AAD:
-
Disorders of amino acid metabolism
- ARG:
-
Argininemia
- ASA:
-
Argininosuccinic aciduria
- BIO:
-
Biotinidase deficiency
- BKT:
-
Beta-ketothiolase deficiency
- BTHA:
-
S beta 0-thalassaemia
- CAH:
-
Congenital adrenal hyperplasia
- CF:
-
Cystic fibrosis
- CH:
-
Primary congenital hypothyroidism
- CITI:
-
Citrullinaemia type I
- CITII:
-
Citrullinaemia type II
- CPT I:
-
Carnitin palmitoyltransferase deficiency type I
- CPT II:
-
Carnitin palmitoyltransferase type II-/Carnitine acylcarnitine transporter deficiency
- CUD:
-
Carnitine uptake defect
- DECR:
-
2,4-Dienoyl-CoA reductase deficiency
- EFTA:
-
European Free Trade Association
- EAHC:
-
Executive Agency for Health and Consumers
- EQA(S):
-
External Quality Assessment (Scheme)
- EUNENBS:
-
European Network of Experts on Newborn Screening
- FAOD:
-
Disorders of fatty acid metabolism
- FYROM:
-
Former Yugoslavian Republic of Macedonia
- GAI:
-
Glutaric acidaemia type I
- GAII:
-
Glutaric acidaemia type II
- GALT:
-
Classical galactosaemia
- HCI:
-
Homocystinuria (CBS deficiency)
- HCSD:
-
Holocarboxylase synthetase deficiency
- Hemo/ HpB:
-
Haemoglobinopathies
- HPLC:
-
High performance liquid chromatography
- HPT I III:
-
Hypermethionaemia types I, III
- ISO:
-
International Standards Organization
- IVA:
-
Isovaleric acidaemia (IVA)/ 2-Methylbutyrylglycinuria
- LCHADD:
-
Long-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency/Trifunctional protein deficiency
- M:
-
Miscellaneous disorders
- MCADD:
-
Medium-chain acyl-CoA dehydrogenase deficiency
- MMA:
-
Malonic acidaemia
- MMACBL:
-
Methylmalonic acidaemia including Cbl A,B C, D defects
- MSUD:
-
Maple Syrup Urine Disease
- NBS:
-
Neonatal (newborn) Screening
- NEQAS:
-
National External Quality Assessment Scheme (UK)
- OA:
-
Disorders of organic acid metabolism
- PA:
-
Propionic acidaemia
- PKU/HPA:
-
Phenylketonuria/Hyperphenylalaninaemia
- QA/QC:
-
Quality assurance/Quality control
- S-S:
-
S,S disease (Sickle cell anaemia)
- SC:
-
S,C disease (Sickle – C disease)
- SCADD:
-
Short-chain acyl-CoA dehydrogenase deficiency
- SCHADD:
-
Medium-short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency
- TYRI:
-
Tyrosinaemia type I
- TYRII_III:
-
Tyrosinaemia types II III
- UDP:
-
UDP-galactose-4-epimerase deficiency
- UK:
-
United Kingdom
- VLCADD:
-
Very long-chain acyl-CoA dehydrogenase deficiency
References
Aymé S, Rodwell C, eds. “2011 Report on the state of the art of rare disease activities in Europe of the European Union Committee of Experts on Rare Diseases - Part I: overview of rare disease activities in Europe and key developments in 2010”, July 2011. http://www.eucerd.eu/upload/file/Reports/2011ReportStateofArtRDActivities.pdf (Accessed 28 Nov 2011)
Bodamer OA, Hoffmann GF, Lindner M (2007) Expanded newborn screening in Europe. J Inherit Metab Dis 30:439–444
Burgard P, Cornel M, Di Filippo F et al. (2011) Report on the practices of newborn screening for rare disorders implemented in Member States of the European Union, Candidate, Potential Candidate and EFTA Countries. http://www.iss.it/cnmr/prog/cont.php?id=1621&lang=1&tipo=64 Accessed 24 Feb 2012
Burgard P, Rupp K, Lindner M et al. (2012) Results of a survey for the evaluation of population newborn screening practices for rare disorders in Europe – From screening laboratory results to treatment, and follow-up, and quality assurance. J Inherit Metab Dis, (in press)
Communication from the Commission to the European Parliament, The Council, The European Economic and Social Committee and The Committee of the Regions on Rare Diseases: Europe’s Challenges (2008). Retrieved from http://ec.europa.eu/health/ph_threats/non_com/docs/rare_com_en.pdf Accessed 22 Nov 2011
Council Recommendation of 8 June 2009 on an action in the field of rare diseases (2009). Retrieved from http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2009:151:0007:0010:EN:PDF) Accessed 22 Nov 2011
Cornel M, Rigter T, Weinreich S, Burgard P, Hoffmann GF, Linder M, Loeber JG, Rupp K, Taruscio D, Vitozzi L (2011) Newborn Screening in Europe; Expert opinion document. http://www.iss.it/cnmr/prog/cont.php?id=1621&lang=1&tipo=64 Accessed24 Feb 2012
ISO 15189 (2007) Medical laboratories -- Particular requirements for quality and competence
ISO 9001 (2008) Quality management systems -- Requirements Retrieved from http://www.iso.org, Accessed 22 Nov 2011
Loeber JG (2007) Neonatal screening in Europe; the situation in 2004. J Inherit Metab Dis 30:430–438
Maastricht Treaty (1992) Treaty establishing the European Community, Art 129. Retrieved from http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:12002E152:EN:HTML, Accessed 6 Dec 2011
National Newborn Screening And Genetics Resource Center (NNSGRC). National Newborn Screening Information System (NNSIS™). Retrieved from http://nnsis.uthscsa.edu/xreports.aspx?XREPORTID=5, Accessed 22 Nov 2011
Watson M, Mann MY, Lloyd-Puryear MA, Rinaldo P, Howell RR (2006) Newborn screening: toward a uniform screening panel and system – executive summary. Pediatrics 117:S296–S307
Wilson JMG, Jungner G (1968) Principles and practice of screening for disease Retrieved from http://www.who.int/bulletin/volumes/86/4/07-050112BP.pdf, Accessed 22 Nov 2011
Zabransky S (2002) Newborn screening for endocrine and metabolic diseases in Europe 2000. Screening J 2:1–14
Acknowledgments
We thank all respondents for contributing their data to the survey.
This work was funded by the European Union contract number 2009 6206 of the Executive Agency for Health and Consumers.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Rodney Pollitt
Rights and permissions
About this article
Cite this article
Loeber, J.G., Burgard, P., Cornel, M.C. et al. Newborn screening programmes in Europe; arguments and efforts regarding harmonization. Part 1 - From blood spot to screening result. J Inherit Metab Dis 35, 603–611 (2012). https://doi.org/10.1007/s10545-012-9483-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-012-9483-0