Abstract
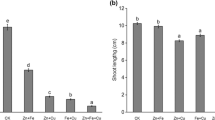
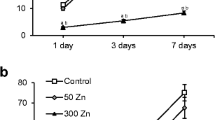
The mechanisms of growth inhibition and antioxidative response were investigated in wheat roots exposed to 300 μM iron together with different zinc concentrations (0, 50, and 250 μM). All Zn concentrations decreased Fe content but increased Zn content in the roots and leaves of Fe-treated seedlings. Compared with Fe stress alone, 50 or 250 μM Zn + Fe treatment stimulated root growth, and increased cell viability but decreased malondialdehyde content, which were correlated with the decreases of total and apoplastic hydrogen peroxide and superoxide anion radical (O2 ·−) content along with apoplastic hydroxyl radical content. Generation of O2 ·− in response to 10 μM diphenylene iodonium suggested that NADPH oxidase activity was lower in Zn + Fe-treated roots than in other roots. In addition, cell wallbound peroxidase, diamine oxidase, and polyamine oxidase in Fe-treated roots were insensitive to Zn addition. Further study showed the stimulation of total superoxide dismutase and glutathione reductase (GR) activities as well as apoplastic catalase, ascorbate peroxidase, and GR in Zn + Fe-stressed roots in comparison with Fe-alone-treated ones. Taken together, Zn could alleviate iron-inhibitory effect on root growth, which might be associated with the decrease of lipid peroxidation, the increase of cell viability and the reductions of reactive oxygen species generation.
Similar content being viewed by others
Abbreviations
- APX:
-
ascorbate peroxidase
- CAT:
-
catalase
- DAO:
-
diamine oxidase
- DPI:
-
diphenylene iodonium
- EDTA:
-
ethylenediaminetetraacetic acid
- GR:
-
glutathione reductase
- H2O2 :
-
hydrogen peroxide
- MDA:
-
malondialdehyde
- NADPH:
-
nicotinamide adenine dinucleotide
- NBT:
-
nitrobluetetrazolium
- O2 ·− :
-
superoxide anion radical
- ·OH:
-
hydroxyl radical
- PAO:
-
polyamine oxidase
- PBS:
-
phosphate buffer solution
- PM:
-
plasma membrane
- POD:
-
peroxidase
- PVP:
-
polyvinylpyrrolidone
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
References
Achary, V.M.M., Jena, S., Panda, K.K., Panda, B.B.: Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L.. — Ecotoxicol. Environ. Safety 70: 300–310, 2008.
Achary, V.M.M., Patnaik, A.R., Panda, B.B.: Oxidative biomarkers in leaf tissue of barley seedlings in response to aluminum stress. — Ecotoxicol. Environ. Safety 75: 16–26, 2012.
Aebi, H.: Catalase in vitro. — Method Enzymol. 105: 121–126, 1984.
Asthir, B., Duffus, C.M., Smith, R.C., Spoor, W.: Diamine oxidase is involved in H2O2 production in the chalazal cells during barley grain filling. — J. exp. Bot. 53: 677–682, 2002.
Auh, C.K., Murphy, T.M.: Plasma membrane redox enzyme is involved in the synthesis of O2 •- and H2O2 by Phytophthora elicitor-stimulated rose cells. — Plant Physiol. 107: 1241–1247, 1995.
Azooz, M.M., Abou-Elhamed, M.F., Al-Fredan, M.A.: Biphasic effect of copper on growth, proline, lipid peroxidation and antioxidant enzyme activities of wheat (Triticum aestivum cv. Hasaawi) at early growing stage. — Aust. J. Crop Sci. 6: 688–694, 2012.
Bradford, M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. — Anal. Biochem. 72: 248–254, 1976.
Chen, L.M., Lin, C.C., Kao, C.H.: Copper toxicity in rice seedlings: changes in antioxidative enzyme activities, H2O2 level, and cell wall peroxidase activity in roots. — Bot. Bull. Acad. sin. 41: 99–103, 2000.
Cherif, J., Mediouni, C., Ammar, W.B., Jemal, F.: Interactions of zinc and cadmium toxicity in their effects on growth and in antioxidative systems in tomato plants (Solarium lycopersicum). — J. environ. Sci. 23: 837–844, 2011.
Clabeaux, B.L., Navarro, D.A., Aga, D.S., Bisson, M.A.: Combined effects of cadmium and zinc on growth, tolerance, and metal accumulation in Chara australis and enhanced phytoextraction using EDTA. — Ecotoxicol. Environ. Safety 98: 236–243, 2013.
Córdoba-Pedregosa, M.C., Villalba, J.M., Córdoba, F., González-Reyes, J.A.: Changes in intracellular and apoplastic peroxidase activity, ascorbate redox status, and root elongation induced by enhanced ascorbate content in Allium cepa L.. — J. exp. Bot. 56: 685–694, 2005.
Cvikrová, M., Gemperlová, L., Dobrá, J., Martincová, O., Prásil, I., Gubis, J., Vanková, R.: Effect of heat stress on polyamine metabolism in proline-over-producing tobacco plants. — Plant Sci. 182: 49–58, 2012.
De Oliveira-Jucoski, G., Cambraia, J., Riveiro, C., Alves-De Oliveira, J., Oliveira-De Paula, S., Oliva, M.A.: Impact of iron toxicity on oxidative metabolism in young Eugenia uniflora L. plants. — Acta Physiol. Plant. 35: 1645–1657, 2013.
Dhindsa, R.S., Matowe, W.: Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. — J. exp. Bot. 32: 79–91, 1981.
Dos Santos, W.D., Ferrarese, M.L.L., Nakamura, C.V., Mourão, K.S.M., Mangolin, C.A., Ferrarese-Filho, O.: Soybean (Glycine max) root lignification induced by ferulic acid. The possible mode of action. — J. chem. Ecol. 34: 1230–1241, 2008.
Dunand, C., Crèvecoeur, M., Penel, C.: Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. — New Phytol. 174: 332–341, 2007.
Fariduddin, Q., Yusuf, M., Hayat, S., Ahmad, A.: Effect of 28-homobrassinolide on antioxidant capacity and photosynthesis in Brassica juncea plants exposed to different levels of copper. — Environ. exp. Bot. 66: 418–424, 2009.
Finger-Teixeira, A., Ferrarese, M.L.L., Soares, A.R., De Silva, D., Ferrarese-Filho, O.: Cadmium-induced lignification restricts soybean root growth. — Ecotoxicol. Environ. Safety 73: 1959–1964, 2010.
Greger, M.: Metal availability and bioconcentration in plants. — In: Hagemeyer, J. (ed.): Heavy Metals Stress in Plants. From Molecules to Ecosystems. Pp. 1–27. Springer, Berlin - Heidelberg 1999.
Halliwell, B., Gutteridge, J., Aruoma, O.I.: The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. — Anal. Biochem. 165: 215–219, 1987.
Halliwell, B., Gutteridge, J.M.C.: Free Radicals in Biology and Medicine. — Clarendon Press, Oxford 1993.
Halliwell, B., Gutteridge, J.M.C.: The chemistry of free radicals and related ‘reactive species’. — Free Radicals Biol. Med. 3: 220, 1999.
Israr, M., Jewell, A., Kumar, D., Sahi, S.V.: Interactive effects of lead, copper, nickel and zinc on growth, metal uptake and antioxidative metabolism of Sesbania drummondii. — J. Hazard. Mater. 186: 1520–1526, 2011.
Karuppanapandian, T., Moon, J.C., Kim, C., Manoharan, K., Kim, W.: Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. — Aust. J. Crop. Sci. 5: 709–725, 2011.
Kranner, I., Roach, T., Beckett, R.P., Whitaker, C., Minibayeva, F.V.: Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum. — J. Plant Physiol. 167: 805–811, 2010.
Lee, T.M., Lin, Y.H.: Changes in soluble and cell wall-bound peroxidase activities with growth in anoxia-treated rice (Oryza sativa L.) coleoptiles and roots. — Plant Sci. 106: 1–7, 1995.
Li, X.N., Ma, H.Z., Jia, P.X., Wang, J., Jia, L.Y., Zhang, T.G., Yang, Y.L., Chen, H.J., Wei, X.: Responses of seedling growth and antioxidant activity to excess iron and copper in Triticum aestivum L.. — Ecotoxicol. Environ. Safety 86: 47–53, 2012a.
Li, X.N., Yang, Y.L., Zhang, J., Jia, L.Y., Li, Q.X., Zhang, T.G., Qiao, K.X., Ma, S.C.: Zinc induced phytotoxicity mechanism involved in root growth of Triticum aestivum L.. — Ecotoxicol. Environ. Safety 86: 198–203, 2012b.
Liu, N., Lin, Z.F., Mo, H.: Metal (Pb, Cd, and Cu)-induced reactive oxygen species accumulations in aerial root cells of the Chinese banyan (Ficus microcarpa). — Ecotoxicology 21: 2004–2011, 2012.
Ma, B., Gao, L., Zhang, H., Gui, J., Shen, Z.: Aluminuminduced oxidative stress and changes in antioxidant defenses in the roots of rice varieties differing in Al tolerance. — Plant Cell Rep. 31: 687–696, 2012.
Maheshwari, R., Dubey, R.S.: Nickel-induced oxidative stress and the role of antioxidative defense in rice seedlings. — Plant Growth Regul. 59: 37–49, 2009.
Matsumoto, H., Motoda, H.: Aluminum toxicity recovery processes in root apices. Possible association with oxidative stress. — Plant Sci. 185: 1–8, 2012.
Naik, B.I., Goswami, R.G., Srivastawa, S.K.: A rapid and sensitive colorimetric assay of amine oxidase. — Anal. Biochem. 111: 146–148, 1981.
Nakano, Y., Asada, K.: Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. — Plant Cell Physiol. 22: 867–880, 1981.
Nenova, V.: Effect of iron supply on growth and photosystem II efficiency of pea plants. — Gen. appl. Plant Physiol. 2006 Special issue: 81–90, 2006.
Pasqualini, S., Cresti, M., Del Casino, C., Faleri, C., Frenguelli, G., Tedeschini, E., Ederli, L.: Roles for NO and ROS signalling in pollen germination and pollen-tube elongation in Cupressus arizonica. — Biol. Plant. 59: 735–744, 2015.
Qiao, X.Q., Zheng, Z.Z., Zhang, L.F., Wang, J.H., Shi, G.X., Xu, X.Y.: Lead tolerance mechanism in sterilized seedlings of Potamogeton crispus L.: Subcellular distribution, polyamines and proline. — Chemosphere 120: 179–187, 2015.
Ranieri, A., Castagna, A., Baldan, B., Soldatini, G.F.: Iron deficiency differently affects peroxidase isoforms in sunflower. — J. exp. Bot. 354: 25–35, 2001.
Rao, M.V., Paliyath, G., Ormrod, D.P.: Ultraviolet-B-and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. — Plant Physiol. 110: 125–136, 1996.
Remans, T., Opdenakker, K., Smeets, K., Matgijsen, D., Vangronsveld, J., Cuypers, A.: Metal-specific and NADPH oxidase dependent changes in lipoxygenase and NADPH oxidase gene expression in Arabidopsis thaliana exposed to cadmium or excess copper. — Funct. Plant Biol. 37: 532–544, 2010.
Schaedle, M., Bassham, J.A.: Chloroplast glutathione reductase. — Plant Physiol. 59: 1011–1012, 1977.
Sergiev, I., Alexieva, V.: Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. — Compt. rend. Acad. Bulg. Sci. 51: 121–124, 1997.
Sinha, S., Basant, A.: Iron-induced oxidative stress in a macrophyte: a chemometric approach. — Ecotoxicol. Environ. Safety 72: 585–595, 2009.
Talaat, N.B., Shawky, B.T.: 24-Epibrassinolide ameliorates the saline stress and improves the productivity of wheat (Triticum aestivum L.). — Environ. exp. Bot. 82: 80–88, 2012.
Tamás, L., Dudiková, J., Durceková, K., Halušková, L., Huttová, J., Mistrík, I.: Effect of cadmium and temperature on the lipoxygenase activity in barley root tip. — Protoplasma 235: 17–25, 2009.
Tewari, R.K., Hadacek, F., Sassmann, S., Lang, I.: Iron deprivation-induced reactive oxygen species generation leads to non-autolytic PCD in Brassica napus leaves. — Environ. exp. Bot. 91: 74–83, 2013.
Thounaojam, T.C., Panda. P., Mazumeder, P., Kumar, D., Sharma, G.D., Sahoo, L., Panda, S.K.: Corrigendum to “Excess copper induced oxidative stress and response of antioxidants in rice”. — Plant Physiol. Biochem. 53: 33–39, 2012.
Turhan, E., Aygogan, C., Baykul, A., Akoglu, A., Evrennosoglu, Y., Ergin, S.: Apoplastic antioxidant enzymes in the leaves of two strawberry cultivars and their relationship to cold-hardiness. — Not. Bot. Hort. Agrobot. 40: 114–122, 2012.
Upadhyay, R.K., Panda, S.K.: Zinc reduces copper toxicity induced oxidative stress by promoting antioxidant defense in freshly grown aquatic duckweed Spirodela polyrhiza L.. — J. Hazard. Mater. 175: 1081–1084, 2010.
Vallee, B.L., Falchuk, K.H.: The biochemical basis of zinc physiology. — Physiol. Rev. 73: 79–118, 1993.
Verma, K., Shekhawat, G.S., Sharma, A., Mehta, S.K., Sharma, V.: Cadmium induced oxidative stress and changes in soluble and ionically bound cell wall peroxidase activities in roots of seedling and 3–4 leaf stage plants of Brassica juncea (L.) Czern. — Plant Cell Rep. 27: 1261–1269, 2008.
Wang, J., Li, W.H., Zhang, C.B., Ke, S.S.: Physiological responses and detoxific mechanisms to Pb, Zn, Cu and Cd in young seedlings of Paulownia fortunei. — J. environ. Sci. 22: 1916–1922, 2010.
Wen, J.F., Deng, M.H., Gong, M.: Cd2+ stress induces two waves of H2O2 accumulation associated with ROSgenerating system and ROS-scavenging system in cultured tobacco cells. — Aust. J. Crop Sci. 6: 846–853, 2012.
Yang, H.Y., Shi, G.X., Wang, H.X., Xu, Q.S.: Involvement of polyamines in adaptation of Potamogeton crispus L. to cadmium stress. — Aquat. Toxicol. 100: 282–288, 2010.
Zanardo, D.I.L., Lima, R.B., Ferrarese, M.L.L., Bubna, G.A., Ferrarese-Filho, O.: Soybean root growth inhibition and lignification induced by p-coumaric acid. — Environ. exp. Bot. 66: 25–30, 2009.
Zhao, H.J., Wu, L.Q., Chai, T.Y., Zhang, Y.X., Tan, J.J., Ma, S.W.: The effects of copper, manganese and zinc on plant growth and elemental accumulation in the manganesehyperaaccumulator Phytolacca americana. — J. Plant Physiol. 169: 1243–1252, 2012.
Zhou, Q.: The measurement of malondialdehyde in plants. — In: Zhou, Q. (ed.): Methods in Plant Physiology. Pp. 173–174. Agricultural Press, Beijing 2001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgments: This work was financially supported by the National Natural Science Foundation of China (Nos. 31470464, 31360094, 31460142), and the Science and Technology Plan Project of Gansu Province (144FKCA059). The first two authors contributed equally to the paper.
Rights and permissions
About this article
Cite this article
Ma, T., Duan, X.H., Yang, Y.Y. et al. Zinc-alleviating effects on iron-induced phytotoxicity in roots of Triticum aestivum . Biol Plant 61, 733–740 (2017). https://doi.org/10.1007/s10535-017-0720-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10535-017-0720-0