Abstract
The structure of lipid A from lipopolysaccharide of Phyllobacterium trifolii PETP02T, a nitrogen-fixing symbiotic bacterium, was studied. It was found that the lipid A backbone was composed of two 2,3-diamino-2,3-dideoxy-d-glucose (GlcpN3N) residues connected by a β-(1 → 6) glycosidic linkage, substituted by galacturonic acid (GalpA) at position C-1 and partly decorated by a phosphate residue at C-4′ of the non-reducing GlcpN3N. Both diaminosugars were symmetrically substituted by 3-hydroxy fatty acids (14:0(3-OH) and 16:0(3-OH)). Ester-linked secondary acyl residues [i.e. 19:0cyc and 28:0(27-OH) or 28:0(27-4:0(3-OMe))] were located in the distal part of lipid A. A high similarity between the lipid A of P. trifolii and Mesorhizobium was observed and discussed from the perspective of the genetic context of both genomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Phyllobacterium was originally described by Knösel (1962), mainly on the basis of phenotypic features of bacteria developing within leaf nodules of tropical ornamental plants. The genus comprised two species, Phyllobacterium myrsinacearum and Phyllobacterium rubiacearum (Knösel 1984), which were merged under the emended description (Mergaert et al. 2002). Currently, the genus Phyllobacterium belongs to the family Phyllobacteriaceae in the order Rhizobiales of the class alpha-Proteobacteria and contains ten species: P. myrsinacearum (Mergaert et al. 2002), P. trifolii (Valverde et al. 2005), P. catacumbae (Jurado et al. 2005), P. bourgognense, P. ifriqiyense, P. leguminum, P. brassicacearum (Mantelin et al. 2006), P. endophyticum (Flores-Fèlix et al. 2013), P. loti (Sánchez et al. 2014), and P. sophorae (Jiao et al. 2015).
Phyllobacterium trifolii PETP02T is the type strain of the species. It has been isolated from nodules of Trifolium pratense belonging to natural clover plants of north-west Spain. A comparison of the 16S rRNA gene sequence of P. trifolii indicated that it is closely related to the members of the genus Mesorhizobium. These bacteria can establish an effective symbiosis with Trifolium and Lupinus, plants that differ with regard to the nodule morphology. This property is rare among symbiotic bacteria. The P. trifolii nodD gene sequence shows high similarity (99%) to the homologous genes of Rhizobium etli CFN32T and Ochrobactrum sp. LUP21, bacteria inducing a determinate type of root nodules on respective legumes, whereas nodules observed on Trifolium, the major macrosymbiont of P. trifolii, represent the indeterminate type (Valverde et al. 2005; Trujillo et al. 2005). The signal molecules (Nod factors) of Rhizobium leguminosarum bv. Trifolii and Bradyrhizobium, typical microsymbionts of clover and lupine, respectively, possess different fine structures. This evidence suggests that Trifolium and Lupinus do not select their microsymbionts strictly and that the host plant receptors could be unspecific (D’Haeze and Holsters 2002; Soulemanov et al. 2002; Schlaman et al. 2006; Renier et al. 2011). Like most Gram-negative bacteria, P. trifolii synthesizes lipopolysaccharide (LPS). The LPS molecules occupy at least 75% of the bacterial cell surface and are composed of three distinct domains: lipid A, core oligosaccharide, and O-specific polysaccharide (O-PS). The domains differ in terms of their structure and biosynthesis pathways (Silipo et al. 2010). Lipid A is a main constituent of the outer leaflet of the outer membrane (OM) and anchors entire LPS in OM through electrostatic and hydrophobic interactions, while the carbohydrate domains of LPS are oriented outwards (Raetz and Whitfield 2002). P. trifolii PETP02T synthesizes mainly the smooth (S) form of LPS, and its O-PS structure has been described (Zamlynska et al. 2015). The strain synthesized two types of O-polysaccharides, containing hexa- and disaccharide repeats, respectively. The proper structure of the entire LPS determines appropriate integrity and flexibility of the outer membrane, essential for the correct morphology and functionality of bacteroids—the endosymbiotic form of rhizobia in which nitrogen fixation takes place. LPS delays or completely blocks the hypersensitivity reaction (HR) induced by rhizobia and suppresses systemic acquired resistance (SAR) during bacterial infection of root nodules (Dow et al. 2000; Albus et al. 2001; Menezes and Jared 2002; Mathis et al. 2005). For a long time, it was believed that lipid A was not important for development of an effective symbiosis. However, the data published during recent years have shown that mutants in genes encoding lipid A biosynthesis were more sensitive to pH and osmolarity changes, grew slowly, and revealed delayed nodule development. Also, the symbiotic effectiveness of these mutants, measured by nitrogen fixation abilities, was considerably reduced (Vedam et al. 2004; Ferguson et al. 2005; Hagg et al. 2009; Choma et al. 2017).
The structure of rhizobial lipids A differ considerably from the model one of Escherichia coli. The enterobacterial glucosamine-based hexa-acylated lipid A has endotoxic activity and is the most potent agonist of innate immunity in humans (Zähringer et al. 1994; Beutler and Rietschel 2003). Respective lipids A from Rhizobium and Sinorhizobium genera contain backbones based on glucosamine and 2-aminogluconate (Bhat et al. 1994; Que et al. 2000a, b; Kannenberg and Carlson 2001; Jeyaretnam et al. 2002; Gudlavalleti and Forsberg 2003; Ferguson et al. 2005, 2006), whereas the disaccharide backbone in other nodule-forming bacteria is composed exclusively of 2,3-diamino-2,3-dideoxy-d-glucose (GlcpN3N) (Choma and Sowiński 2004; Komaniecka et al. 2010; Choma et al. 2012; Brown et al. 2013; Komaniecka et al. 2014; Silipo et al. 2014). Sugar backbones of rhizobial lipids A are substituted by amide- and ester-linked 3-hydroxy fatty residues, whose hydroxyl groups can be acylated by other non-polar and (ω-1)-hydroxy/oxo very long chain fatty acid (VLCFA). For example, 27-hydroxyoctacosanoic acid is most often found in lipids A of members of alpha-rhizobia (except for the Azorhizobium genus) and is considered as chemotaxonomic marker of this group of bacteria (Bhat et al. 1991a, b). These very long chain fatty acids could be partially acylated by 3-hydroxybutyric or 3-methoxybutyric acids or by one or two hopanoid residues in some bradyrhizobial lipids A (Komaniecka et al. 2014; Silipo et al. 2014; Choma et al. 2017).
The structures of lipids A isolated from symbiotic bacteria classified outside of the Rhizobiaceae and Bradyrhizobiaceae families, such as that of the Phyllobacterium genus, have been studied sporadically, up to now. The aim of this article was to fill the gap and describe the structure of lipid A from P. trifolii PETP02T in detail.
Materials and methods
Bacterial culture conditions and lipopolysaccharide isolation
The P. trifolii PETP02T strain was obtained as a kind gift from Dr. E. Velazquez, from the University of Salamanca, Spain. The bacteria were cultivated for 7 days in a liquid Tryptone Yeast medium at 28 °C with aeration by vigorous shaking. The bacteria were harvested and the cell pellet was washed twice with saline. Bacterial mass was subjected to delipidation and enzymatic digestion procedures according to the method described in detail by Choma et al. (2012). The lipopolysaccharide was extracted from degraded cells using a hot 45% phenol/water method (Westphal and Jann 1965; Johnson and Perry 1976) and purified by ultracentrifugation, as described by Zamlynska et al. (2015).
Lipid A isolation and O-deacylation
Lipid A was cleaved from LPS by mild acid hydrolysis (aq 1% acetic acid, 100 °C, 3 h). After cooling, the hydrolysate was converted to the two-phase Bligh-Dyer system and the chloroform phase containing the lipid A portion was separated by centrifugation (4000 × g, 15 min, 20 °C), collected, and washed with the water phase from a freshly prepared two-phase Bligh–Dyer mixture (Que et al. 2000a; Choma et al. 2012). The lipid A preparation obtained was stored at −20 °C in chloroform/methanol (2:1, v/v). Lipid A was O-deacylated by incubation of a 1 mg sample in chloroform/methanol/1 M aqueous NaOH, 2:3:1 (v/v/v), for 1 h, at room temperature, according to the method described previously (Que-Gewirth et al. 2004; Komaniecka et al. 2014). Partial lipid A O-deacylation was performed using 25% ammonium hydroxide (Lukasiewicz et al. 2010).
Chemical analyses of lipid A
The sugar composition was established by hydrolysis of lipid A with 4 M HCl (100 °C, 4 h) and conversion of liberated monosaccharides into (amino)alditol acetates (Sawardeker et al. 1965). The absolute configuration of the monosaccharides was established by analysis of trimethylsilylated R-(-)-butyl glycosides according to a modified procedure developed by Gerwig et al. (1978). The fatty acid composition was established after methanolysis of lipid A (2 M HCl/MeOH, 85 °C, 18 h) and conversion of the obtained hydroxy fatty acid methyl esters into trimethylsilyl derivatives. 3-Hydroxy fatty acids were converted to l-phenylethylamides of 3-methoxy derivatives as described previously (Rietschel et al. 1976, Wollenweber et al. 1980) for analysis of the absolute configuration. Sugars and fatty acid derivatives were analysed using a gas chromatograph (Agilent Technologies, instrument 7890A) connected to a mass selective detector (Agilent Technologies MSD 5975C, inert XL EI/CI) (GLC-MS), using helium as a carrier gas. The chromatograph was equipped with a HP-5MS column (30 m × 0.25 mm). The temperature program was as follows: 150 °C for 5 min raised to 310 °C (5 °C min−1), and the final temperature was maintained for 10 min. l-Phenylethylamides of 3-methoxy fatty acid derivatives were also analysed isothermally (270 °C) using the same column. Heptadecanoic acid and 3-hydroxy tetradecanoic acids were used as standards for quantitative determination of fatty acids.
NMR spectroscopy
The NMR spectra were recorded at 30 °C using a Varian Inova 500 instrument and a standard Varian software. The sample was dissolved in a mixture of CDCl3/CD3OD (1:1, v/v) with a drop (5 µl) of D2O. 1D (1H and 31P NMR) and 2D NMR spectra (1H,1H COSY, DQF-COSY, TOCSY, NOESY, ROESY) were recorded. Proton chemical shifts were measured in relation to TMS as an internal standard (δH 0.00). Phosphorous chemical shifts were measured relative to an external standard of 85% (v/v) phosphoric acid at δP 0.00 p.p.m.
MALDI-TOF MS and MS/MS spectrometry
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF–MS) was performed using a Waters SYNAPT G2-Si HDMS instrument (Waters Corporation, Milford, MA, USA) equipped with a 1 kHz Nd:YAG laser system. Acquisition of the data was performed using MassLynx software version 4.1 SCN916 (Waters Corporation, Wilmslow, United Kingdom). Spectra were recorded in positive and negative ion polarities. For MS/MS experiments, isolated precursor ions were fragmented using collision voltage of 60 V. Data were collected for 120 s for each ion separately. Mass spectra were assigned with a multi-point external calibration using red phosphorous (Sigma).
The lipid A sample was dissolved in chloroform/methanol (2:1, v/v) at a concentration of 10 µg/µl and one microliter of the sample was transferred into the target plate wells covered with a thin matrix film. The matrix solution was prepared from 2′,4′,6′-trihydroxyacetophenone (THAP) (200 mg/ml in methanol) mixed with nitrocellulose (NC) (15 mg/ml, suspended in 2-propanol/acetone (1:1, v/v)) in proportion of 4:1 (v/v), as previously described by Silipo et al. (2005).
Bioinformatics tools
Standard BLASTP (with the cut-off E-value of 10−5) was used in searching for putative proteins engaged in the biosynthetic pathway of Phyllobacterium lipid A. Mesorhizobium loti MAFF 303099 protein sequences were used as queries in BLASTP searches against five Phyllobacterium strains registered in the Genomes OnLine Database. Individual protein sequences were then compared across their entire span with an on-line Global Alignment tool (using the Needleman-Wunsch algorithm) provided by the National Center for Biotechnology Information (NCBI).
Results
Structural analysis of the lipid A preparation from P. trifolii
Delipidated P. trifolii cells were extracted with 45% hot phenol in water and LPS was found mainly in the water phase. The lipid A fraction was obtained by mild hydrolysis of LPS using 1% acetic acid in water. Galacturonic acid (GalpA) and GlcpN3N were identified as the only sugar components of lipid A. Their absolute configuration was shown to be d. Fatty acid analysis revealed the presence of 14:0(3-OH), 15:0(3-OH), 16:0(3-OH), 17:0(3-OH), and 18:0(3-OH) acids (Table 1). All of them were amide-linked and had the d absolute configuration. d-3-Hydroxy acids are characteristic components of all lipopolysaccharides described so far (Rietschel 1976). 3-Hydroxytetradecanoic and 3-hydroxyhexadecanoic acids were the main 3-hydroxy fatty acids in lipid A. Two long chain (ω-1)-hydroxy fatty acids (28:0(27-OH) and 30:0(29-OH)) as well as their oxo-analogues (28:0(27-oxo) and 30:0(29-oxo)) were present as ester-linked substituents of lipid A. Among ester-linked acids, non-polar fatty acids (16:0, 18:0, 18:1, and 19:0cyc) were found. The lactobacillic acid (19:0cyc) was the most abundant. This component was identified based on its chromatographic properties (retention time), compared with an authentic lactobacillic methyl ester. Cyclopropane ring-containing fatty acids give characteristic series of artificial components after methanolysis (Orgambide et al. 1993). All these artificial fatty acids were found in the solvolysed (in methanolic HCl) lipid A preparation (data not shown).
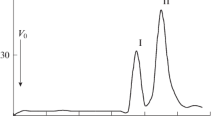
An aliquot of lipid A was analysed by negative ion mode MALDI-TOF spectrometry (Fig. 1). Lipid A showed significant heterogeneity due to the 3-OH-fatty acid variability (Table 2). The heaviest group of ions was found between m/z at 2354.6 and 2405.0 showing the presence of a hexaacylated lipid A species composed of two GlcpN3N residues, uronic acid, phosphate residue, 28:0(27-(4:0(3-OCH3)) acyloxyacyl residue, 19:0cyc, and 3-hydroxy-acyl chains with different length (Δ mass = 14 amu). Ions representing lipid A species devoid of phosphate (Δ mass = 80 amu) and 3-O-methoxybutyryl (Δ mass = 100 amu) were placed on the other side of the spectrum (signals between m/z at 2159.6 and 2229.7). The middle part of the spectrum is the most crowded region. This group of ions comprises lipid A species modified with phosphate but not with 3-O-methoxybutyryl and vice versa (3-O-methoxybutyryl-containing, but deprived of phosphate). A number of lipid A molecules bearing a 28:0(27-oxo) residue were found in this region. They were always decorated with phosphate. Negative and positive ions derived from the O-deacylated lipid A are depicted in Fig. 2a, b. Negative ions representing O-deacylated lipid A molecules are in the m/z range from 1225.8 to 1665.0 (Fig. 2a, b). The less abundant group of ions around m/z at 1855 in the negative ion mode spectrum can be treated as not fully O-deacylated lipid A (Fig. 2a). The mass difference of 278.2 amu pointed to the lactobacillic residue attached to the primary 3-hydroxy fatty acid in lipid A. Three groups of ions (around m/z at 1600, 1498, and 1322) can be classified to (i) complete, (ii) dephosphorylated, and (iii) deprived of both phosphate and galacturonic acid residue O-deacylated lipid A, respectively (Fig. 2a, b and Table 2). The difference in the mass of the intact and O-deacylated lipid A molecules confirmed the previously mentioned findings that both 28:0(27-OH/oxo) and 19:0cyc constitute ester linked secondary residues.
Although MALDI is recognized as a soft ionization technique, it is known that, with the desorption of intact molecules/ions, in-source fragmentation occurs. Very informative B+ type lipid A ions can be found in the positive ion mode spectra of intact as well as O-deacylated lipid A preparations (Fig. 3a, b and Table 2). They were helpful in determination of the fatty acid distribution. The ions at m/z 1340.160 and 1420.136 unequivocally indicated that distal GlcpN3N was partly modified with phosphate (Δ mass = 80 amu). In addition, asymmetric distribution (4 + 2) of acyl residues on the disaccharide backbone of intact lipid A was deduced from the analysis of these ions. Thus, the ion at m/z 1340.160 can be interpreted as a tetra-acylated B1 + fragment bearing N-linked 16:0(3-OH) and 14:0(3-OH), and ester-linked lactobacillic and 27-oxooctacosanoic acids. The spectrum of O-deacylated lipid A included B1 + type ions as well. Ions at m/z 641.509 and 721.475 represented phosphorylated and unphosphorylated lipid A fragments composed of two fatty acids attached to GlcpN3N oxonium ions. Both these ions were accompanied by two mono- and doubly dehydrated product ions (Fig. 3b). Taking together the data from the analyses of intact and O-deacylated lipid A, it can be concluded that lipid A primary fatty acids were distributed symmetrically. MS/MS analyses were performed to assign the exact primary fatty acid positions at the reducing as well as non-reducing GlcpN3N. A set of ions was chosen from the O-deacylated P. trifolii lipid A MALDI TOF MS spectrum. The fragmentation pattern of the ion at m/z 1498.12 was selected to illustrate our results (Fig. 4a, b). This sodiated ion represents tetraacylated (exclusively with primary fatty acids) and devoid of phosphate species of P. trifolii lipid A (Table 2). After loss of GalpA and water residues (176.04 and 18.01 amu, respectively), the precursor ion generated an ion at m/z 1304 containing a double bond between C-1 and C-2 of reducing GlcpN3N. The enamine to imine tautomerization of the compound resulted in elimination of aldehyde from the C-2 amide-bound fatty acid containing a free β-OH group (Choma et al. 2012; Silipo et al. 2014; Di Lorenzo et al. 2017). From two possibilities of GlcpN3N substitution with 3-OH fatty residues, only this one was represented and yielded a final ion at m/z 451.29 (Fig. 4a, b). The presence of this signal in the spectrum (and the absence of an ion peak at m/z 641.51) supported the placement of 16:0(3-OH) at C-2 and 14:0(3-OH) at C-3 of reducing as well as non-reducing GlcpN3N of the P. trifolii lipid A. The presence of other ions in the spectrum shown in Fig. 4b was explained by taking into consideration different fragmentation mechanisms as well (Fig. 4a).
Positive ion mode MALDI TOF MS spectra. a Part of the spectrum containing B1 + ions derived from intact P. trifolii PETP02T lipid A and b part of the spectrum containing B1 + ions derived from O-deacylated lipid A (with 1 M NaOH). Most of the selected ions in the spectra (marked in bold) are described in Table 2
According to Silipo et al. (2014), an ion corresponding to the pentaacylated lipid A, containing a VLCFA as the only secondary residue, is the best for determining the VLCFA position in the lipid A molecule. Intact lipid A from P. trifolii contained many hexaacylated species but there were few pentaacylated molecules (Fig. 1). Therefore, we used 25% ammonium hydroxide to remove partly and randomly O-linked fatty acids from the lipid A. Following the methodology proposed by Silipo et al. (2014), an ion at m/z 2000.34 with the composition (GlcN3N)2GalA1P1[C14:0(3-OH)]2[C16:0(3-OH)]2[28:0(27-OH)]1Na (P: represents a phosphate residue) was selected. Two B-type ions (B2-type at m/z 1806.4 and B1 at m/z 1165.9) were found (data not shown). Only the heavier one was accompanied by an ion originating from loss of ketene (∆ mass = 212.2 amu). These results allowed us to conclude that VLCFA (28:0(27-OH)) was linked to the hydroxyl of the primary fatty acid attached to position C-2′ of the lipid A backbone. These results are in agreement with the hitherto published VLCFA containing lipid A structures (Choma et al. 2017).
MALDI-TOF analysis clearly indicated that P. trifolii lipid A was very similar to its M. huakuii counterpart. Moreover, with respect to their backbones, both lipids A were identical. Thus, we decided to reduce the scope of the NMR analysis to 1H,1H homonuclear correlations sufficient to determine the anomeric configuration and the linkage positions within the lipid A backbone. The NMR data are listed in Table 3. The proton chemical shifts were assigned based on 1H,1H DQF-COSY, TOCSY, and NOESY spectra. Three proton signals were identified in the anomeric region of 1H NMR indicating that the P. trifolii lipid A backbone was built up of three sugar residues. Three spin systems: A, α-d-GalpA; B, α-d-GlcpN3N; and C, β-d-GlcpN3N were described as well. The sequence of monosaccharides was established in the NOESY experiment by observation of the following interresidue correlations: A-1/B-1 (δ 5.19/5.04), C-1/B-6 (δ 4.39/3.76), and C-1/B-6′ (δ 4.39/3.84). The small values of 3 J H-1,H-2 coupling constants (<3 Hz) for GalpA (A) and GlcpN3N (B) indicated an α-anomeric configuration of both residues. Thus, they were linked via an α-(1↔1)-glycosidic bond. Similar proton spectra were published for M. huakuii lipid A (Choma and Sowiński 2004).
The 31P-NMR spectrum of the intact lipid A revealed a prominent signal with a chemical shift of 0.199 ppm observed in the solvent being a mixture of chloroform and methanol (proportion 2:1, v:v, respectively). These properties were indicative of the presence of phosphomonoester.
Based on all collected data, the following structure for lipid A being the lipophilic part of P. trifolii lipopolysaccharide was proposed (Fig. 5).
Genetic background of lipid A biosynthesis pathway in Phyllobacterium species
In order to validate the experimental data related to the structure of P. trifolii PETP02T lipid A obtained in MALDI-TOF mass spectrometry and NMR spectroscopy, in silico analyses of Phyllobacterium genomic sequences were performed for identification of putative genes encoding proteins engaged in lipid A biosynthesis. Although there is no available annotated genome sequence of P. trifolii, there are five high quality permanent draft genome sequences of strains belonging to the genus Phyllobacterium, among the genome sequencing projects registered in GOLD (Genomes OnLine Database) (Pagani et al. 2012). These strains comprise Phyllobacterium sp. OV277 (GOLD Project ID: Gp0136754), Phyllobacterium sp. UNC302MFCol5.2 (GOLD Project ID: Gp0039813), Phyllobacterium sp. YR620 (GOLD Project ID: Gp0136755), Phyllobacterium sp. YR531 (GOLD Project ID: Gp0012211), and Phyllobacterium sp. CL33Tsu (GOLD Project ID: Gp0115127). All five Phyllobacterium strains were isolated, cultured, and sequenced as part of three independent plant–microbe associated projects. Therefore, we used these genomic resources in searching for candidate genes of the P. trifolii lipid A biosynthetic pathway.
A comparative sequence analysis of the 16S rRNA gene indicated that P. trifolii is closely related to the members of the genus Mesorhizobium (Valverde et al. 2005). Our results have confirmed that the structure of P. trifolii lipid A is indeed similar (the backbone structure is even identical) to some mesorhizobial lipids A, i.e. M. huakuii IFO 15243T and M. loti MAFF 303099 (Choma and Sowiński 2004; Brown et al. 2013). Thus, protein sequences of model rhizobial strain M. loti MAFF 303099 were used as queries in BLAST sequence similarity searching the draft genome sequences of the phyllobacteria, and then respective protein sequences were compared across their entire length with Needleman-Wunsch Global Align. Using this approach, we were able to recognize a set of putative genes coding for common enzymes required for the biosynthesis of lipid A (lpxA, lpxC, lpxD, lpxH, lpxB, lpxK, and kdtA (waaA)) as well as encoding specific enzymes involved in the structural modifications of lipid A found in some Gram-negative bacteria (lpxE, rgtF, and acpXL-lpxXL). Moreover, genes coding for enzymes converting GlcpN to GlcpN3N, necessary for biosynthesis of the lipid A disaccharide backbone and characteristic for bacteria from the genera Mesorhizobium, Azorhizobium and Bradyrhizobium (gnnA and gnnB), were identified (Table 4). Putative ORFs of the tested phyllobacterial species shared significant sequence similarity with the respective M. loti MAFF 303099 proteins (Table 4), strongly suggesting their common evolutionary ancestry—homology. We were not able to predict the putative homolog of α-(1↔1)-GalpA transferase (rgtF gene) in the genome of Phyllobacterium sp. YR531. We found ORFs coding for putative 4′-phosphatase in the genomes of four Phyllobacterium strains (Ga0115491_102664, BR48DRAFT_1751, Ga0115492_1841, and Ga0073264_1478 in Phyllobacterium spp. OV277, UNC302MFCol5.2, YR620, and CL33Tsu, respectively), sharing 50–51% sequence similarity with the LpxF protein of R. leguminosarum bv. viciae 3841 (RL_RS08140), but we cannot identify homologs of RgtD, a putative 4′-GalA transferase of R. leguminosarum bv. viciae 3841 (RL_RS03600). Both LpxF and RgtD are involved in transfer of the GalpA residue to lipid A during biosynthesis of LPS in R. leguminosarum bv. viciae 3841 (Brown et al. 2013).
The putative genes described display substantial conservation of organization (synteny and collinearity) among the tested phyllobacteria and in comparison to M. loti MAFF 303099 (Table 5). This was especially apparent with lpxD, lpxA, and lpxB, as well as kdtA and lpxK gene clusters, and the highly conserved acpXL-lpxXL region, typical for bacteria possessing lipid A molecules modified with VLCFAs (Choma et al. 2017). Similar to M. loti, the phyllobacterial lpxC and lpxH were not clustered with other genes related to LPS biosynthesis (Table 5).
There were noticeable differences found in the location of putative genes responsible for the transamination reaction of GlcpN (gnnA and gnnB), and structural modifications of GlcpN3N (lpxE and rgtF). In the case of phyllobacteria, orthologs of gnnA and gnnB are located close to each other but separately from the lpxA, lpxB, lpxD gene cluster, similar to Bradyrhizobium japonicum and Brucella melitensis (Sweet et al. 2004). In contrast, in M. loti MAFF 303099, the putative orthologs of these genes are separated both from each other and from the lpxA, lpxB, lpxD cluster. The rgtF and lpxE genes of M. loti MAFF303099 were clustered together with a putative rgtE gene (encoding putative bactoprenyl-phosphate GalpA transferase) located upstream rgtF. Brown et al. (2013) suggested a functional relationship of these genes in the biosynthesis of lipid A α-(1↔1)-GalA. Interestingly, in all the tested phyllobacterial species, the putative rgtE and rgtF orthologs were neighboured, while the putative homolog of lpxE was distantly located from the above-mentioned ORFs.
Discussion
In this study, we have described the structure of P. trifolii PETP02T lipid A, which contains a trisaccharide carbohydrate backbone. This backbone comprises two d-GlcpN3N connected by a β-(1 → 6) glycosidic linkage and a d-GalpA residue at position C-1. The substitution of the reducing end of lipid A by α-(1↔1)-d-GalpA is unusual among bacteria and has been only described in lipids A from a few representatives of Gram-negative bacteria, including an associative diazotroph—Azospirillum lipoferum (Choma and Komaniecka 2008), and symbiotic bacteria—M. huakuii (Choma and Sowiński 2004), M. loti (Brown et al. 2013), a stalk-forming Caulobacter crescentus (Smit et al. 2008), and a thermophilic bacterium Aquifex pyrophilus (Plötz et al. 2000). The GlcpN3N disaccharide of C. crescentus and A. pyrophilus is bis-galacturonosylated with GalpA located at positions C-1 and C-4′ (Plötz et al. 2000; Smit et al. 2008), whereas the A. lipoferum backbone does not possess the substituent at the reducing end of GlcpN (Choma and Komaniecka 2008). The P. trifolii lipid A trisaccharide backbone is partially phosphorylated at position C-4′ and has the same structure as that of M. huakuii IFO15243T (Choma and Sowiński 2004). One can speculate that this may be related to the lack of an rgtD homolog in the genomes of the tested phyllobacterial species. The presence of phosphate, GlcpN3N, and GalpA has also been reported in lipid A from R. loti, but with α-(1→4′)-GalpA, not α-(1↔1)-GalpA (Russa et al. 1995). The amino groups of both GlcpN3N of P. trifolii lipid A are symmetrically substituted by 3-hydroxy fatty acids, among which 14:0(3-OH) and 16:0(3-OH) dominate. Moreover, MS/MS data supported the placement of 16:0(3-OH) at C-2 and 14:0(3-OH) at C-3 positions of reducing as well as non-reducing GlcpN3N of the lipid A. Although the backbones of lipids A synthesized by phyllobacteria and mesorhizobia are identical, the substituting primary fatty acids are significantly different. This fact can be explained by the different basic metabolisms of both genera of bacteria. While the Mesorhizobium species synthesize and incorporate branched chain fatty acids into lipids (including lipids A), Phyllobacterium spp. produce predominantly straight chain fatty acids for cellular purposes (Tighe et al. 2000; Choma and Sowiński 2004; Valverde et al. 2005). As can be seen, the biosynthetic pathway of VLCFAs is not subject to this rule. Mesorhizobium as well as Phyllobacterium produces the same VLCFAs. Because of the distribution of ester-linked fatty acids (19:0cyc and 28:0-(27-OH/oxo)) restricted to the distal GlcpN3N, the entire lipid A is asymmetrically acylated, resembling E. coli and mesorhizobial lipids A (Raetz and Whitfield 2002; Choma and Sowiński 2004; Brown et al. 2013). This pattern of fatty acid distribution can be described by the formula 4 + 2. Hydroxyl groups at β positions of the primary fatty acids were additionally acylated by VLCFA and lactobacillic acid. The 27-hydroxyoctacosanoic acid could be partially acylated with 3-methoxybutyric acids. Lactobacillic acid is frequently found as a constituent of bacterial phospholipids. Especially phospholipids extracted from bacteria harvested in the stationary phase of growth are rich in this fatty acid as well as other cyclopropane fatty acids. To the best of our knowledge, cyclopropane fatty acids have not been found yet to be a component of LPS/lipid A. It can be assumed that, similar to phospholipids (Gronan and Cronan 1997), LPS containing unsaturated 18:1ω7 (vaccenic) acyl residue in the lipid A moiety undergoes modification in the bacterial outer membrane. This issue also remains to be resolved in future studies.
As mentioned above, we have found structural resemblance of lipid A of P. trifolii and Mesorhizobium and this observation seems to be reflected at the genomic level. Putative ORFs predicted for LPS biosynthesis in the phyllobacteria shared significant sequence similarity and overall similar gene organization with M. loti, with only minor differences. Further genetic analyses are required to answer the question how these differences affect the structure of P. trifolii lipopolysaccharide.
References
Albus U, Baier R, Holst O, Pühler A, Niehaus K (2001) Suppression of an elicitor-induced oxidative burst in Medicago on sativacell-cultures by Sinorhizobium meliloti lipopolysaccharides. New Phytol 151:597–606
Beutler B, Rietschel ET (2003) Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol 3:169–176
Bhat UR, Carlson RW, Busch M, Mayer H (1991a) Distribution and phylogenetic significance of 27-hydroksyoctacosanoic acid in lipopolysaccharides from bacteria belonging to the alpha-2 subgroup of proteobacteria. Int J Syst Bacteriol 41:213–2017
Bhat UR, Mayer H, Yokota A, Hollingsworth RI, Carlson RW (1991b) Occurrence of lipid A variants with 27-hydroxyoctacosanoic acid in lipopolysaccharides from members of the family Rhizobiaceae. J Syst Bacteriol 173:2155–2159
Bhat UR, Forsberg LS, Carlson RW (1994) The structure of the lipid A component of Rhizobium leguminosarum bv. phaseoli lipopolysaccharide. A unique non-phosphorylated lipid A containing 2-amino-2-deoxy-gluconate, galacturonate, and glucosamine. J Biol Chem 269:14402–14410
Brown DB, Muszynski A, Carlson RW (2013) Elucidation of a novel lipid A α-(1,1)-GalA transferase gene (rgtF) from Mesorhizobium loti: Heterologous expression of rgtF causes Rhizobium etli to synthesize lipid A with α-(1,1)-GalA. Glycobiology 23:546–558
Choma A, Komaniecka I (2008) Characterization of a novel lipid A structure isolated from Azospirillum lipoferum lipopolysaccharide. Carbohydr Res 343:799–804
Choma A, Sowiński P (2004) Characterization of Mesorhizobium huakuii lipid A containing both d-galacturonic acid and phosphate residues. Eur J Biochem 271:1310–1322
Choma A, Komaniecka I, Turska-Szewczuk A, Danikiewicz W, Spolnik G (2012) Structure of lipid A from a stem-nodulating bacterium Azorhizobium caulinodans. Carbohydr Res 352:126–136
Choma A, Komaniecka I, Zebracki K (2017) Structure, biosynthesis and function of unusual lipids A from nodule-inducing and N2-fixing bacteria. Biochim Biophys Acta Mol Cell Biol Lipids 1862:196–209
D’Haeze W, Holsters M (2002) Nod factor structures, responses, and perception during initiation of nodule development. Glycobiology 12(6):79R–105R
Di Lorenzo F, Palmigiano A, Al Bitar-Nehme S, Sturiale L, Duda KA, Gully D, Lanzetta R, Giraud E, Garozzo D, Bernardini ML, Molinaro A, Silipo A (2017) The lipid A from Rhodopseudomonas palustris strain BisA53 LPS possesses a unique structure and low immunostimulant properties. Chemistry 23:3637–3647
Domon B, Costello C (1988) A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconjugate J 5:397–409
Dow JM, Newman MA, Von Roepenack E (2000) The induction and modulation of plant defense responses by bacterial lipopolysaccharides. Annu Rev Phytopathol 38:241–261
Ferguson GP, Datta A, Carlson RW, Walker GC (2005) Importance of unusually modified lipid A in Sinorhizobium stress resistance and legume symbiosis. Mol Microbiol 56:68–80
Ferguson GP, Jansen A, Marlow VL, Walker GC (2006) BacA-mediated bleomycin sensitivity in Sinorhizobium meliloti is independent of the unusual lipid A modification. J Bacteriol 188:3143–3148
Flores-Felix J-D, Carro L, Velazquez E, Valverde A, Castillo CE, Garcia-Fraile P, Rivas R (2013) Phyllobacterium endophyticum sp. nov., isolated from nodules of Phaseolus vulgaris. Int J Syst Evol Microbiol 63:821–826
Gerwig GJ, Kamerling IP, Viegenthart JFG (1978) Determination of the d and l configuration of neutral monosaccharides by high-resolution capillary g.l.c. Carbohydr Res 62:349–357
Gronan DW, Cronan JE (1997) Cyclopropane ring formation in membrane lipids of bacteria. Microbiol Mol Biol Rev 61:429–441
Gudlavalleti SK, Forsberg LS (2003) Structural characterization of the lipid A component of Sinorhizobium sp. NGR234 rough and smooth form lipopolysaccharide. Demonstration that the distal amide-linked acyloxyacylresidue containing the long chain fatty acid is conserved in Rhizobium and Sinorhizobium sp. J Biol Chem 278:3957–3968
Hagg AF, Wehmeier S, Beck S, Marlow VL, Fletcher V, James EK, Ferguson GP (2009) The Sinorhizobium melilotii Lpx and AcpXL proteins play important roles in bacteroid development within alfalfa. J Bacteriol 191:4681–4686
Jeyaretnam B, Glushka J, Kolli VSK, Carlson RW (2002) Characterization of a novel lipid-A from Rhizobium species Sin-1. A unique lipid-A structure that is devoid of phosphate and has a glycosyl backbone consisting of glucosamine and 2-aminogluconic acid. J Biol Chem 277:41802–41810
Jiao YS, Yan H, Ji ZJ, Liu YH, Sui XH, Zhang XX, Wang ET, Chen WX, Chen WF (2015) Phyllobacterium sophorae sp. nov., a symbiotic bacterium isolated from root nodules of Sophora flavescens. Int J Syst Evol Microbiol 65:399–406
Johnson KG, Perry MB (1976) Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol 22:29–34
Jurado V, Laiz L, Gonzalez JM, Hernandez-Marine M, Valens M, Saiz-Jimenez C (2005) Phyllobacterium catacumbae sp. nov., a member of the order ‘Rhizobiales’ isolated from Roman catacombs. Int J Syst Evol Microbiol 55:1487–1490
Kannenberg EL, Carlson RW (2001) Lipid A and O-chain modifications cause Rhizobium lipopolysaccharides to become hydrophobic during bacteroid development. Mol Microbiol 39(2):379–391
Knösel DH (1962) Prüfung von Bakterien auf Fähigkeit zur Sternbildung. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg II 116:79–100
Knösel DH (1984) Genus IV. Phyllobacterium (ex Knösel 1962) nom. rev. (Phyllobacterium Knösel 1962, 96). In: Krieg NR, Holt JG (eds) Bergey’s Manual of Systematic Bacteriology, Vol 1. Williams and Wilkins, Baltimore, pp 254–256
Komaniecka I, Choma A, Lindner B, Holst O (2010) The structure of a novel neutral lipid A from the lipopolysaccharide of Bradyrhizobium elkanii containing three mannose units in the backbone. Chem Eur J 16:2922–2929
Komaniecka I, Choma A, Mazur A, Duda KA, Lindner B, Schwudke D, Holst O (2014) Occurrence of an unusual hopanoid-containing lipid A among lipopolysaccharides from Bradyrhizobium species. J Biol Chem 289:35644–35655
Lukasiewicz J, Jachymek W, Niedziela T, Kenne L, Lugowski C (2010) Structural analysis of the lipid A isolated from Hafnia alvei 32 and PCM 1192 lipopolysaccharides. J Lipid Res 51:564–574
Mantelin S, Fischer-Le Saux M, Zakhia F, Bena G, Bonneau S, Jeder H, de Lajudie P, Cleyet-Marel JC (2006) Emended description of the genus Phyllobacterium and description of four novel species associated with plant roots: Phyllobacterium bourgognense sp. nov., Phyllobacterium ifriqiyense sp. nov., Phyllobacterium leguminum sp. nov. and Phyllobacterium brassicacearum sp. nov. Int J Syst Evol Microbiol 56:827–839
Mathis R, Van Gijsegem F, De Rycke R, D’Haeze W, Van Maelsaeke E, Anthonio E, Van Montagu M, Holsters M, Vereecke D (2005) Lipopolysaccharides as a communication signal for progression of legume endosymbiosis. PNAS 102:2655–2660
Menezes H, Jared C (2002) Immunity in plants and animals: common ends through different means using similar tools. Comp Biochem Physiol Part C 132:1–7
Mergaert J, Cnockaert MC, Swings J (2002) Phyllobacterium myrsinacearum (subjective synonym Phyllobacterium rubiacearum) emend. Int J Syst Evol Microbiol 52:182–1823
Orgambide GG, Reusch RN, Dazzo FB (1993) Methoxylated fatty acids reported in Rhizobium isolates arise from chemical alterations of common fatty acids upon acid-catalyzed transesterification procedures. J Bacteriol 175:4922–4926
Pagani I, Liolios K, Jansson J, Chen IM, Smirnova T, Nosrat B, Markowitz VM, Kyrpides NC (2012) The Genomes OnLine Database (GOLD) v. 4: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Res 40:D571–D579
Plötz BM, Lindner B, Stetter KO, Holst O (2000) Characterization of a novel lipid A containing d-galacturonic acid that replaces phosphate residues. The structure of the lipid A of the lipopolysaccharide from the hyperthermophilic bacterium Aquifex pyrophilus. J Biol Chem 275:11222–11228
Que NLS, Lin SH, Cotter RJ, Raetz CRH (2000a) Purification and mass spectrometry of six lipid A species from the bacterial endosymbiont Rhizobium etli—demonstration of a conserved distal unit and a variable proximal portion. J Biol Chem 275:28006–28016
Que NLS, Ribeiro AA, Raetz CRH (2000b) Two-dimensional NMR spectroscopy and structures of six lipid A species from Rhizobium etli CE3—detection of an acyloxyacyl residue in each component and origin of the aminogluconate moiety. J Biol Chem 275:28017–28027
Que-Gewirth NLS, Ribeiro AA, Kalb SR, Cotter RJ, Bulach DM, Adler B, Girons IS, Werts C, Raetz CRH (2004) A methylated phosphate group and four amide-linked acyl chains in Leptospira interrogans lipid A. The membrane anchor of an unusual lipopolysaccharide that activates TLR2. J Biol Chem 279:25420–25429
Raetz CRH, Whitfield C (2002) Lipopolisaccharide endotoxins. Annu Rev Biochem 71:635–700
Renier A, Maillet F, Fardoux J, Poinsot V, Giraud E, Nouwen N (2011) Photosynthetic Bradyrhizobium sp. strain ORS285 synthesizes 2-O-Methylfucosylated lipochitooligosaccharides for nod gene–dependent interaction with Aeschynomene plants. Mol Plant Microbe Interact 24:1440–1447
Rietschel ET (1976) Absolute configuration of 3-hydroxy fatty acids present in lipopolysaccharide from various bacterial groups. Eur J Biochem 64:423–428
Russa R, Urbanik-Sypniewska T, Lindström K, Mayer H (1995) Chemical characterization of two lipopolysaccharide species isolated from Rhizobium loti NZP2213. Arch Microbiol 163:345–351
Sánchez M, Ramírez-Bahena MH, Peix A, Lorite MJ, Sanjuán J, Velázquez E, Monza J (2014) Phyllobacterium loti sp. nov. isolated from nodules of Lotus corniculatus. Int J Syst Evol Microbiol 64:781–786
Sawardeker JS, Sloneker JH, Jeanes AR (1965) Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Anal Chem 37:1602–1604
Schlaman HRM, Olsthoorn MMA, Harteveld M, Dörner L, Djordjevic MA, Thomas-Oates JE, Spaink HP (2006) The production of species-specific highly unsaturated fatty acyl-containing LCOs from Rhizobium leguminosarum bv. Trifolii is stringently regulated by nodD and involves the nodRL genes. Mol Plant Microbe Interact 19:215–226
Silipo A, Molinaro A, Sturiale L, Dow JM, Erbs G, Lanzetta R, Newman MA, Parrilli M (2005) The elicitation of plant innate immunity by lipooligosaccharide of Xanthomonas campestris. J Biol Chem 280:33660–33668
Silipo A, De Castro C, Lanzetta R, Parrilli M, Molinaro A (2010) Lipopolysaccharides. In: Konig H, Claus H, Varma A (eds) Prokaryotic cell wall compounds: structure and biochemistry. Springer, Heidelberg, pp 133–154
Silipo A, Vitiello G, Gully D, Sturiale L, Chaintreuil C, Fardoux J, Gargani D, Lee HI, Kulkarni G, Busset N, Marchetti R, Palmigiano A, Moll H, Engel R, Lanzetta R, Paduano L, Parrilli M, Chang WS, Holst O, Newman DK, Garozzo D, D’Errico G, Giraud E, Molinaro A (2014) Covalently linked hopanoid-lipid A improves outer-membrane resistance of a Bradyrhizobium symbiont of legumes. Nat Commun 5:5106
Smit J, Kaltashov IA, Cotter RJ, Vinogradov E, Perry MB, Haider H, Qureshi N (2008) Structure of a novel lipid A obtained from the lipopolysaccharide of Caulobacter crescentus. Innate Immun 14:25–36
Soulemanov A, Prithiviraj B, Carlson RW, Jeyaretnam B, Smith DL (2002) Isolation and characterization of the major nod factor of Bradyrhizobium japonicum strain 532C. Microbiol Res 157:25–28
Sweet CR, Ribeiro AA, Raetz CR (2004) Oxidation and transamination of the 3″-position of UDP-N-acetylglucosamine by enzymes from Acidithiobacillus ferrooxidans. Role in the formation of lipid a molecules with four amide-linked acyl chains. J Biol Chem 279:25400–25410
Tighe SW, de Lajudie P, Dipietro K, Lindström K, Nick G, Jarvis BD (2000) Analysis of cellular fatty acids and phenotypic relationships of Agrobacterium, Bradyrhizobium, Mesorhizobium, Rhizobium and Sinorhizobium species using the Sherlock Microbial Identification System. Int J Syst Evol Microbiol 50:787–801
Trujillo ME, Willems A, Abril A, Planchuelo A-M, Rivas R, Ludena D, Mateos PF, Martinez-Molina E, Velaquez E (2005) Nodulation of Lupinus albus by strains Ochrobactrum lupinii sp. Nov. Appl Environ Microbiol 71:1318–1327
Valverde A, Velázquez E, Fernández-Santos F, Vizcaíno N, Rivas R, Mateos PF, Martínez-Molina E, Igual JM, Willems A (2005) Phyllobacterium trifolii sp. nov., nodulating Trifolium and Lupinus in Spanish soils. Int J Syst Evol Microbiol 55:1985–1989
Vedam V, Haynes JG, Kannenberg EL, Carlson RW, Sherrier DJ (2004) A Rhizobium leguminosarum lipopolysaccharide lipid A mutant induces nitrogen-fixing nodules with delayed and defective bacteroid formation. Mol Plant Microbe Interact 17:283–291
Westphal O, Jann K (1965) Bacterial lipopolysaccharide. Extraction with phenol-water and further application of the procedure. In: Whistler RL (ed) Methods in carbohydrate chemistry, vol 5. Academic Press, New York, pp 83–91
Wollenweber HW, Rietschel ET, Hofstad T, Wentraub A, Lindberg AA (1980) Nature, type of linkage, quantity, and absolute configuration of (3-Hydroxy) fatty acids in lipopolysaccharides from Bacteroides fragilis NCTC 9343 and related strains. J Bacteriol 144:898–903
Zähringer U, Lindner B, Rietschel ET (1994) Molecular structure of lipid A, the endotoxic center of bacterial lipopolysaccharides. Adv Carbohydr Chem Biochem 50:211–276
Zamlynska K, Komaniecka I, Turska-Szewczuk A, Pac M, Choma A (2015) The O-specific polysaccharides from Phyllobacterium trifolii LPS contain 3-C-methyl-d-rhamnose. Carbohydr Res 409:25–29
Acknowledgements
The spectrometer Waters SYNAPT G2-Si HDMS was purchased from the European Union Funds, Operating Programme Infrastructure and Environment (Project Number: UDA-POiS.13.01-045/08) and the GC–MS instrument was purchased from the Eastern Poland European Funds for the years 2007–2013 (Project Number: POPW.01.03.00-06-009/11-00). This work was financially supported by the BS/UMCS research program (Grant Numbers: BS-P-11-010-16-2-01 (for K.Z., I.K., A.S.-B. A.Ch.) and BS-M-11-010-16-2-07 (for K.Z.)). The authors thank Dr. Pawel Sowinski (Intercollegiate NMR Laboratory, Department of Chemistry, Gdansk University of Technology, Poland) for recording the NMR spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zamlynska, K., Komaniecka, I., Zebracki, K. et al. Studies on lipid A isolated from Phyllobacterium trifolii PETP02T lipopolysaccharide. Antonie van Leeuwenhoek 110, 1413–1433 (2017). https://doi.org/10.1007/s10482-017-0872-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-017-0872-0