Abstract
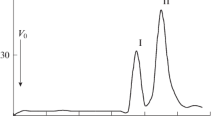
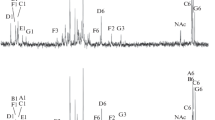
Phenol-water extraction of Rhizobium loti NZP2213 cells allowed a simultaneous isolation of two structurally different lipopolysaccharides, from the aqueous (LPS-W) and phenol (LPS-P) phase that differed in their sodium doexycholate-PAGE pattern and composition. LPS-W showed a profile indicating an R-type LPS; LPS-P had a cluster of poorly resolved bands in the high-molecular-weight region. LPS-P contained large amounts of 6-deoxy-l-talose (6dTal), and a small amount of 2-O-methyl-6-deoxy-talose (molar ratio ≈30:1), both of which were completely absent in LPS-W. Methylation analysis gave only one major product, 2,4-di-O-methyl-6dTal, indicating that the O-chain is composed of a homopolymer of 1,3-linked 6dTal, having the methylated 6dTal (2-O-me-6dTal) probably localized at the non-reducing end of the O-chain. This homopolymeric O-chain was additionally O-acetylated, as evidenced by GC-MS and by 13C NMR analysis. The lipid A moieties of both LPS-W and LPS-P showed almost identical composition, with six, different 3-OH fatty acids and with two, so far not described, long-chain 4-oxo-fatty acids, all being amide-linked, and with 27-OH-28:0 as the main ester-linked fatty acid. Lipid A was of the lipid ADAG-type, i.e., having a (phosphorylated) 2,3-diamino-2,3-dideoxy-d-glucose-containing lipid A backbone. Lipid ADAG is widespread among species of the α-2 group of Proteobacteria, but has so far not been encountered in any other rhizobial or agrobacterial species.
Similar content being viewed by others
References
Bhat UR, Kontrohr T, Mayer H (1987) Structure of Shigella sonnei lipid A. FEMS Microbiol Lett 40:189–192
Bhat UR, Carlson RW, Busch M, Mayer H (1991a) Distribution and phylogenetic significance of 27-hydroxy-octacosanoic acid in lipopolysaccharides from bacteria belonging to the alpha-2 subgroup of proteobacteria. Int J Syst Bacteriol 41:213–217
Bhat UR, Mayer H, Yokota A, Hollingsworth RI, Carlson RW (1991b) Occurrence of lipid A variants with 27-hydroxyoctacosanoic acid in lipopolysaccharides from mutants of the family Rhizobiaceae. J Bacteriol 173:2155–2159
Bhat UR, Forsberg LS, Carlson RW (1994) Structure of lipid A component of Rhizobium leguminosarum bv. phaseoli lipopolysaccharide J Biol Chem 269:14402–14410
Busch M, O'Connor SP, Brenner DJ, Mayer H (1991) Lipopolysaccharide composition of two strains of cat scratch disease-associated bacteria (abstract). Meeting of Vereinigung für allgemeine und Angewandte Mikrobiologie, p 419
Choma A, Russa R, Urbanik-Sypniewska T, Mayer H (1994) Characterization of lipopolysaccharides of Rhizobium huakuii, the microsymbiont of Astragalus (abstract). Interaction between microorganisms and plants, University of Lublin, p 64
Gerwig GJ, Kamerling JP, Vliegenthart JF (1978) Determination of the d and l configuration of neutral monosaccharides by high-resolution capillary GLC. Carbohydr Res 62:349–357
Hollingsworth RI, Lill-Elghanian D (1990) Endotoxin structure and activity: an old problem from a new perspective. In: Nowotny A, Spitzer JJ, Ziegler EJ (eds) Cellular and molecular aspects of endotoxin reactions. Excerpta Medica, Amsterdam New York Oxford, pp 73–84
Hotter GS, Scott DB (1991) Exopolysaccharide mutants of Rhizobium loti are fully effective on a determinate nodulating host but are ineffective on an undeterminate nodulating host. J Bacteriol 173:851–859
Jarvis BCW, Pankhurst CE, Patel JJ (1982) Rhizobium, loti, a new species of legume root nodule bacteria. Int J Syst Bacteriol 32: 378–380
Katayama Y, Hiraishi A, Kuraishi H (1994) Phylogenetic studies on the strains of Thiobacillus species based on 16S rRNA sequences (abstract). Annu Meeting Jpn. Soc Biosci Biotechnol Agrochem, p 336
Komuro T, Galanos C (1988) Analysis of Salmonella lipopolysaccharides by sodium deoxycholate-polyacrylamide gel electrophoresis. J Chromatogr 450:381–387
Krauss JH, Weckesser J, Mayer H (1988) Electrophoretic analysis of lipopolysaccharides of purple nonsulfur bacteria. Int J Syst Bacteriol 38:157–163
Lindström K, Lehtomäki S (1988) Metabolic properties, maximum growth temperature and phage sensitivity of Rhizobium sp. (Galega) compared with other fast-growing rhizobia. FEMS Microbiol Lett 50:277–287
Lorkiewicz Z, Derylo M, Russa R, Skorupska A, Urbanik-Sypniewska T (1993) Rhizobium leguminosarum biovar trifolii mutants altered in surface structures that are defective in nodulation or nitrogen fixation. Acta Microbiol Polonica 42: 219–234
Lowry OH, Roberts NR, Kuner KY, Wu NL, Farr AL (1954) The quantitative histochemistry of brain. 1. Chemical methods. J Biol Chem 207:1–17
Mayer H, Masoud H, Urbanik-Sypniewska T, Weckesser J (1989a) Lipid A composition and phylogeny of gram-negative bacteria. Bull Jpn Fed Cult Collections 5:19–25
Mayer H, Krauß JH, Urbanik-Sypniewska T, Puvanesarajah V, Stacey G, Auling G (1989b) Lipid A with 2,3-diamino-2,3-dideoxy-glucose in lipopolysaccharides from slow-growing members of Rhizobiaceae and from Pseudomonas carboxydovorans. Arch Microbiol 151:111–116
Noël KD, Vandenbosch KA, Kulpaca B (1986) Mutations in Rhizobium phaseoli that lead to arrested development of infection threads. J Bacteriol 168:1392–1401
Russa R, Urbanik-Sypniewska T, Choma A, Mayer H (1991) Identification of 3-deoxy-lyxo-2-heptulosaric acid in the core region of lipopolysaccharides from Rhizobiaceae. FEMS Microbiol Lett 84:337–344
Shashkov AS, Campos-Portuguez S, Kochanowski H, Yokota A, Mayer H (1995) the structure of the O-specific polysaccharide from Thiobacillus sp IFO 14570 with three different diaminopyranoses froming the repeating unit. Carbohydr Res (in press)
Shibuya N, Amano K, Azuma JI, Nishihara T, Kitamura Y, Noguchi T, Koga R (1991) 6-Deoxy-d-talan and 6-deoxy-l-talan. Novel serotype-specific polysaccharide antigens from Actinobacillus actinomycetemcomitans. J Biol Chem 266: 16318–16323
Sonesson A, Moll H, Jantzen E, Zähringer U (1993) Long-chain α-hydroxy-(ω-1)-oxo fatty acids and α-hydroxy-1, ω-dioic fatty acids are cell wall constituents of Legionella (L. jordanis, L-maceachernii and L. micdadei). FEMS Microbiol Lett 106: 315–320
Tharanathan RN Yokota A, Rau H, Mayer H (1993) Isolation and chemical characterization of lipopolysaccharides from four Mycoplana species (M. bullata, M. ramosa and M. dimorpha). Arch Microbiol 159:445–452
Tsai CM, Frasch CE (1982) A sensitive silver-staining for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem 119:115–119
Vincent M (1970) A manual for the practical study of root-nodule bacteria. International biological programme, handbook no. 15. Blackwell Oxford Edinburgh
Weckesser J, Mayer H (1988) Different lipid A types in lipopolysaccharides of phototrophic and related non-phototrophic bacteria. FEMS Microbiol Rev 54:143–154
Weibgen U, Russa R, Yokota A, Mayer H (1993) Taxonomic significance of the lipopolysaccharide composition of the three biovars of Agrobacterium tumefaciens. Syst Appl Microbiol 16: 177–182
Westphal O, Jann K (1965) Bacterial lipopolysaccharides, extraction with phenol-water and further application of the procedure. Methods Carbohydr Chem 5:83–91
Wilkinson SG (1988) Gram-negative bacteria. In: Ratledge C, Wilkinson SG (eds) Microbial lipids, vol 1. Academic Press, New York London, pp 299–488
Wollenweber HW, Rietschel ET (1990) Analysis of lipopolysaccharide (lipid A) fatty acids. J Microbiol Methods 11:195–211
Yanagi M, Yamasato K (1993) Phylogenetic analysis of the family Rhizobiaceae and related bacteria by sequencing of 16S rRNA gene using PCR and DNA sequences. FEMS Microbiol 107: 115–120
Yokota A, Sakane T (1991) Taxonomic significance of fatty acids compositions in whole cells and lipopolysaccharides in Rhizobiaceae. Inst Ferment Oska Res Commun 15:57–75
Yokota A, Sakane T, Ophel K, Sawada H (1993) Further studies on the cellular fatty acid composition of Rhizobium and Agrobacterium species. Inst Ferment Osaka Res Commun 16: 86–94
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Russa, R., Urbanik-Sypniewska, T., Lindström, K. et al. Chemical characterization of two lipopolysaccharide species isolated from Rhizobium loti NZP2213. Arch. Microbiol. 163, 345–351 (1995). https://doi.org/10.1007/BF00404207
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00404207