Abstract
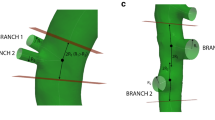
Recent research has revealed that angiotensin II-induced abdominal aortic aneurysm in mice can be related to medial ruptures occurring in the vicinity of abdominal side branches. Nevertheless a thorough understanding of the biomechanics near abdominal side branches in mice is lacking. In the current work we present a mouse-specific fluid–structure interaction (FSI) model of the abdominal aorta in ApoE−/− mice that incorporates in vivo stresses. The aortic geometry was based on contrast-enhanced in vivo micro-CT images, while aortic flow boundary conditions and material model parameters were based on in vivo high-frequency ultrasound. Flow waveforms predicted by FSI simulations corresponded better to in vivo measurements than those from CFD simulations. Peak-systolic principal stresses at the inner and outer aortic wall were locally increased caudal to the celiac and left lateral to the celiac and mesenteric arteries. Interestingly, these were also the locations at which a tear in the tunica media had been observed in previous work on angiotensin II-infused mice. Our preliminary results therefore suggest that local biomechanics play an important role in the pathophysiology of branch-related ruptures in angiotensin-II infused mice. More elaborate follow-up research is needed to demonstrate the role of biomechanics and mechanobiology in a longitudinal setting.




Similar content being viewed by others
References
Arruda, E. M., et al. A 3-dimensionl constitutive model for the large stretch behavior of rubber elastic materials. J. Mech. Phys. Solids 41(2):389–412, 1993.
Bersi, M. R., et al. Disparate changes in the mechanical properties of murine carotid arteries and aorta in response to chronic infusion of angiotensin-II. Int. J. Adv. Eng. Sci. Appl. Math. 4(4):228–240, 2013.
Bols, J., et al. A computational method to assess the in vivo stresses and unloaded configuration of patient-specific blood vessels. J. Comput. Appl. Math. 246:10–17, 2013.
Bols, J., et al. Inverse modelling of image-based patient-specific blood vessels: zero pressure geometry and in vivo stress incorporation. ESAIM—Math. Model. Num. Anal. 47(4):1059–1075, 2013.
Bols, J., et al. Unstructured hexahedral mesh generation in complex vascular structures using a grid-based approach. Comput. Methods Biomech. Biomed. Eng., 2014, under review.
Broisat, A., et al. Assessing low levels of mechanical stress in aortic atherosclerotic lesions from apolipoprotein E−/− mice—brief report. Arterioscler. Thromb. Vasc. Biol. 31(5):1007–1010, 2011.
Caro, C. G. Discovery of the role of wall shear in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29(2):158–161, 2009.
Chandra, S., et al. Fluid-structure interaction modeling of abdominal aortic aneurysms: the impact of patient-specific inflow conditions and fluid/solid coupling. J. Biomech. Eng. 135(8):081001, 2013.
Chen, G., et al. Impaired erythrocyte deformability in transgenic HO-1G143H mutant mice. Transgenic Res. 24(1):173–178, 2015.
Cheng, C., et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113(23):2744–2753, 2006.
Collins, M. J., et al. Mechanical properties of suprarenal and infrarenal abdominal aorta: Implications for mouse models of aneurysms. Med. Eng. Phys. 33(10):1262–1269, 2011.
Daugherty, A., et al. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Investig. 105(11):1605–1612, 2000.
Daugherty, A., et al. Antagonism of AT2 receptors augments Angiotensin II-induced abdominal aortic aneurysms and atherosclerosis. Br. J. Pharmacol. 134(4):865–870, 2001.
Degroote, J. Development of Algorithms for the Partitioned Simulation of Strongly Coupled Fluid-structure Interaction Problems, in Faculty of Engineering Ghent University, Ghent pp. 108–110, 2010.
Degroote, J., et al. Stability of a coupling technique for partitioned solvers in FSI applications. Comput. Struct. 86(23–24):2224–2234, 2008.
Degroote, J., et al. Performance of a new partitioned procedure versus a monolithic procedure in fluid-structure interaction. Comput. Struct. 87(11–12):793–801, 2009.
Endo, S., et al. Flow patterns and preferred sites of atherosclerotic lesions in the human aorta—II. Abdom. Aorta Biorheol 51(4):257–274, 2014.
Feintuch, A., et al. Hemodynamics in the mouse aortic arch as assessed by MRI, ultrasound, and numerical modeling. Am. J. Physiol. Heart Circ. Physiol. 292(2):H884–H892, 2007.
Ford, M. D., et al. Hemodynamics of the Mouse Abdominal Aortic Aneurysm. J. Biomech. Eng. 133(12):121008, 2011.
Gasser, T. C., et al. A rate-independent elastoplastic constitutive model for biological fiber-reinforced composites at finite strains: continuum basis, algorithmic formulation and finite element implementation. Comput. Mech. 29(4–5):340–360, 2002.
Gavish, L., et al. Low level laser arrests abdominal aortic aneurysm by collagen matrix reinforcement in apolipoprotein E-deficient mice. Lasers Surg. Med. 44(8):664–674, 2012.
Gavish, L., et al. Inadequate reinforcement of transmedial disruptions at branch points subtends aortic aneurysm formation in apolipoprotein-E-deficient mice. Cardiovasc. Pathol. 23(3):152–159, 2014.
Greve, J. M., et al. Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics. Am. J. Physiol. Heart Circ. Physiol. 291(4):H1700–H1708, 2006.
Hoi, Y., et al. Correlation between local hemodynamics and lesion distribution in a novel aortic regurgitation murine model of atherosclerosis. Ann. Biomed. Eng. 39(5):1414–1422, 2011.
Holzapfel, G. A., et al. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J. Elast. 61(1–3):1–48, 2000.
Huo, Y. L., et al. The flow field along the entire length of mouse aorta and primary branches. Ann. Biomed. Eng. 36(5):685–699, 2008.
Ku, D. N., et al. Pulsatile flow and atherosclerosis in the human carotid bifurcation—positive correlation between plaque location and low and oscillating shear-stress. Arteriosclerosis 5(3):293–302, 1985.
Leung, J. H., et al. Fluid structure interaction of patient specific abdominal aortic aneurysms: a comparison with solid stress models. Biomed. Eng. Online 5:33, 2006.
Manning, M. W., et al. Abdominal aortic aneurysms: fresh insights from a novel animal model of the disease. Vasc. Med. 7(1):45–54, 2002.
Rabben, S. I., et al. Ultrasound-based vessel wall tracking: an auto-correlation technique with RF center frequency estimation. Ultrasound Med. Biol. 28(4):507–517, 2002.
Reddy, A. K., et al. Measurement of aortic input impedance in mice: effects of age on aortic stiffness. Am. J. Physiol.-Heart Circ. Physiol. 285:H1464–H1470, 2003.
Reymond, P., et al. Physiological simulation of blood flow in the aorta: Comparison of hemodynamic indices as predicted by 3D FSI, 3D rigid wall and 1D models. Med. Eng. Phys. 35(6):784–791, 2013.
Rissland, P., et al. Abdominal aortic aneurysm risk of rupture: patient-specific FSI simulations using anisotropic model. J. Biomech. Eng. 131(3):031001, 2009.
Saraff, K., et al. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 23(9):1621–1626, 2003.
Scotti, C. M., et al. Wall stress and flow dynamics in abdominal aortic aneurysms: finite element analysis vs. fluid-structure interaction. Comput. Methods Biomech. Biomed. Eng. 11(3):301–322, 2008.
Thompson, S., et al. Hereditary differences in serum proteins of normal mice. Proc. Soc. Exp. Biol. Med. 87(2):315–317, 1954.
Trachet, B., et al. An integrated framework to quantitatively link mouse-specific hemodynamics to aneurysm formation in angiotensin II-infused ApoE−/− mice. Ann. Biomed. Eng. 39(9):2430–2444, 2011.
Trachet, B., et al. The impact of simplified boundary conditions and aortic arch inclusion on CFD simulations in the mouse aorta: a comparison with mouse-specific reference data. J. Biomech. Eng. 133(12):121006–121013, 2011.
Trachet, B., et al. Longitudinal follow-up of ascending versus abdominal aortic aneurysm formation in angiotensin II-infused ApoE−/− mice. Artery Res. 8(1):16–23, 2014.
Trachet, B., et al. Dissecting abdominal aortic aneurysm in ang II-infused mice: suprarenal branch ruptures and apparent luminal dilatation. Cardiovasc. Res. 105(2):213–222, 2015.
Van Doormaal, M., et al. Inputs for subject-specific computational fluid dynamics simulation of blood flow in the mouse aorta. J. Biomech. Eng. 136(10):101008, 2014.
Westerhof, N., et al. An artificial arterial system for pumping hearts. J. Appl. Physiol. 31:776–781, 1971.
Acknowledgments
This research was funded by the Special Research Fund of Ghent University under Grant [BOF10/GOA/005] and by internal funds of the Laboratory of Hemodynamics and Cardiovascular Technology, EPFL. B.T. received a travel grant of the Flemish Fund for Scientific Research. The computational resources and services used in this work were provided by the VSC (Flemish Supercomputer Center), funded by Ghent University, the Hercules Foundation and the Flemish Government—department EWI.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Umberto Morbiducci oversaw the review of this article.
Bram Trachet and Joris Bols contributed equally to this work.
Rights and permissions
About this article
Cite this article
Trachet, B., Bols, J., Degroote, J. et al. An Animal-Specific FSI Model of the Abdominal Aorta in Anesthetized Mice. Ann Biomed Eng 43, 1298–1309 (2015). https://doi.org/10.1007/s10439-015-1310-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1310-y