Abstract
West Nile virus has caused several outbreaks among humans in the Phoenix metropolitan area (Arizona, southwest USA) within the last decade. Recent ecologic studies have implicated Culex quinquefasciatus and Culex tarsalis as the mosquito vectors and identified three abundant passerine birds—great-tailed grackle (Quiscalus mexicanus), house sparrow (Passer domesticus), and house finch (Haemorhous mexicanus)—as key amplifiers among vertebrates. Nocturnal congregations of certain species have been suggested as critical for late summer West Nile virus amplification. We evaluated the hypothesis that house sparrow (P. domesticus) and/or great-tailed grackle (Q. mexicanus) communal roost sites (n = 22 and n = 5, respectively) in a primarily suburban environment were spatially associated with West Nile virus transmission indices during the 2010 outbreak of human neurological disease in metropolitan Phoenix. Spatial associations between human case residences and communal roosts were non-significant for house sparrows, and were negative for great-tailed grackle. Several theories that explain these observations are discussed, including the possibility that grackle communal roosts are protective.
Similar content being viewed by others
Introduction
West Nile virus (WNV; flavivirus: Flaviviridae) became established throughout North America between 1999 and 2004 and is currently endemic in the USA (Kilpatrick 2011). Epidemics of WNV infections with neurologic disease manifestations (i.e., encephalitis, meningitis, acute flaccid paralysis) among humans are sporadic and focal, and occur when ecological conditions support spillover transmission from enzootic transmission cycles. Culex sp. mosquitoes typically serve as vectors, and certain bird species serve as amplifying hosts (Hayes et al. 2005). An outbreak of human WNV neurologic disease occurred in metropolitan Phoenix in the summer of 2010. Epidemiologic studies corroborated the vector roles of Culex quinquefasciatus and Cx. tarsalis mosquitoes within the outbreak region by finding that both proximity of Culex breeding sites and local Culex abundance were identified as risk factors for human WNV disease (Gibney et al. 2012). Ecologic studies evaluated candidate avian amplifying hosts and, using a modified calculation for mosquito inoculation index, determined that great-tailed grackle (Quiscalus mexicanus), house sparrow (Passer domesticus), house finch (Haemorhous mexicanus), and to a lesser extent, mourning dove (Zenaida macroura) were all involved in amplifying WNV (Komar et al. 2013).
Numerous species of birds develop high-titered viremia and are thus competent to transmit WNV to hematophagous mosquitoes (Komar et al. 2003). However, the vertebrate amplifiers within a transmission focus must not only be competent, but also abundant and attractive to vectors (Kilpatrick 2011). High levels of WNV exposure among the four candidate amplifiers were observed: great-tailed grackle, 86%; house sparrow, 51%; house finch, 100%; and mourning dove, 45% (Komar et al. 2013).These four candidate avian amplifiers are all known to roost communally.
Avian behaviors, such as roosting, may influence WNV transmission, although few studies have addressed this topic. For example, Ward et al. (2006)studied the locations of nocturnal roosts selected by Northern cardinal (Cardinalis cardinalis) and American crow (Corvus brachyrhynchos) and estimated that a viremic crow could spread WNV over an area 700-fold larger than that of a viremic cardinal. Other studies have evaluated the effect of communal roosting for a variety of bird species on WNV-infection rates in mosquitoes (Reisen et al. 2009; Diuk-Wasser et al. 2010; Benson et al. 2012). The communal roost studies theorize that avian clusters attract Culex mosquitoes and provide a renewable source of fuel (i.e., WNV-susceptible birds) to amplify WNV. Indeed, Komar et al. (2013) documented that the number of vector-amplifier contacts, as determined by the density of resting Cx. quinquefasciatus and Cx. tarsalis mosquitoes that contained vertebrate blood, was 25-fold and 13-fold higher, respectively, at communal bird roosts compared to matched control sites in suburban Phoenix during the 2010 epidemic.
While numerous studies have investigated environmental risk factors associated with WNV outbreaks [reviewed in Paz and Semenza (2013)], avian behavioral risk factors (such as communal roosting) for human WNV-associated illness have not been evaluated. The increased density of vector-host contacts due to communal roosting behavior of certain birds may escalate the risk of human WNV infections around these nocturnal congregations of amplifying hosts. Therefore, we evaluated the hypothesis that communal bird roost sites were spatially associated with WNV transmission to people and mosquitoes during the 2010 outbreak by analyzing human case data, mosquito infection data, and the location of large congregations of roosting birds.
Methods
Study Area
A rectangular area of southeast Maricopa County measuring 6.4 km (4.0 mi) × 16.1 km (10.0 mi.) was selected because of the cluster of human case residences within the region and accessibility by automobile throughout the region (Fig. 1). Portions of the municipalities of Chandler, Gilbert, and Mesa were included in the study area.
Locations of Avian Communal Roosts
Communal bird roosts were detected by a single observer driving a grid network of approximately 80 miles of major paved roads spaced at 1-mile intervals throughout the 40-square-mile study area during the final 45 min of daylight for each of 10 days while listening and watching for bird congregations. The grid was covered twice during the study period. The flight vectors of flocks were plotted on a map. Triangulation of multiple vectors indicated the locations of nocturnal roosts. The geographic locations of these roost sites were recorded and visited to confirm the presence and species identity of the roosting flocks. Detection of communal roosts was carried out from September 14–20, and from October 26–28, 2010.
Locations of Residences of Human Cases and Non-cases
Geocoded data for case and non-case residences within the study area were obtained from a larger case–control study conducted by CDC and the Maricopa County Department of Public Health (Gibney et al. 2012). Briefly, a case was defined as a resident of the southeast section of Maricopa County (“East Valley”) with laboratory-confirmed WNV disease, as reported to the Maricopa County Department of Public Health. Laboratory confirmation required detection of WNV-reactive IgM antibody or specific WNV RNA in blood or cerebral spinal fluid. A control was defined as an East Valley resident presenting with WNV-like signs and/or symptoms but with a cerebrospinal fluid sample testing negative for WNV-reactive IgM ≥4 days after symptom onset or a serum sample testing negative for WNV-reactive IgM ≥7 days after symptom onset. Human WNV cases occurred from late May through September, and peaked in July. Human cases utilized for the study occurred between May 28 and July 31, 2010.
Mosquito Infection Data
Data from routine mosquito collection sites located within the study area were provided by Maricopa County Environmental Service Vector Control Division and were utilized to estimate WNV-infection rates within the study area. Collection sites were selected independently from locations of avian communal roosts, based mainly on the locations of historical nuisance reports. Cx. quinquefasciatus and Cx. tarsalis collected overnight in CO2-baited CDC light traps (with light bulbs removed) were sorted by species and pooled into groups of up to 50 adult females. Pools were homogenized and tested for WNV infection using the RAMP® WNV Test (Response Biomedical, Vancouver, BC); RAMP values ≥ 100 were considered positive for the detection of WNV antigen. Cumulative WNV-infection rates for the 3-month period May 25–August 28, 2010 were estimated using bias-corrected maximum likelihood for each species and trap location (Biggerstaff 2006).
Geospatial and Statistical Analysis
Geographic coordinates for communal bird roosts and mosquito traps were geocoded using ArcGIS 10.0 (ESRI, Redlands, CA), and mapped in conjunction with geocoded case and non-case residence data. To investigate the spatial associations among communal bird roosts and human WNV case residences, the Euclidean distances between human case and non-case residences and their nearest communal roosts were calculated using Hawth’s Tools in ArcGIS, and compared using exact permutation tests for the differences of means and medians (Lehmann 1991); 95% confidence intervals were calculated using bootstrap resampling methods (Davison and Hinkley 1997; Canty and Ripley 2013). A logistic regression model was used to determine if distance from roost was significantly associated with human infection. For mosquito infection rate analyses, we estimated the infection rates at each trap using pooled binomial methods (Hepworth 2005; Biggerstaff 2006). Potential association between infection rates at mosquito trap locations and their distance to the nearest great-tailed grackle or house sparrow roost were evaluated using pooled binomial regression methods (Xi 2001). Analyses were performed using SAS version 9.1 and R version 2.14.2 (http://www.r-project.org, and using the lme4, coin, boot, and binGroup packages).
Results
Locations of 22 communal house sparrow roosts and 5 communal great-tailed grackle roosts, 20 mosquito trap sites, and 31 human residences (16 cases and 15 non-cases) were mapped within the study area (Fig. 1). These 27 communal roost sites were consistently active during the period of data collection.
Sparrow roosts were typically located in shrubbery of oleander (Nerium oleander) and hosted 20-200 individuals. Grackle roosts were typically in ornamental giant timber bamboo (Bambusa oldhamii). The grackle roosts each contained >500 individuals and small numbers of other blackbird species (family Icteridae) such as cowbirds (Molothrus species) as well as European starling (Sturnus vulgaris). Both the sparrow and grackle utilized ornamental vegetation for roost trees, and thus were always located near human residences and other buildings. Major dove and finch roosts (with >20 individuals) were scarce in the study region. The absence of major dove and finch communal roosts led us to focus on sparrow and grackle communal roosts for the data analysis.
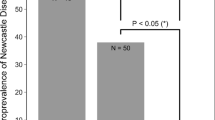
To test for an association between the location of sparrow or grackle communal roost sites and WNV case residences, we compared the mean and median distances between these and the residences of cases and non-cases within the study site (Table 1). A significant difference was detected for grackle roosts but not sparrow roosts. Case residences were significantly farther away from grackle roosts than non-case residences. The logistic regression model also showed that distance from communal grackle roosts was significantly associated with the infection status of case residences (OR = 4.41, 95% CI 1.28–15.16), but sparrow communal roost distance was not significantly associated (OR = 0.46, 95% CI 0.18–1.20).
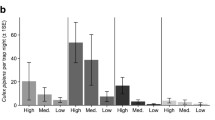
During the period May 25 through Aug 28, 19 of 81 (22.2%) Cx. quinquefasciatus pools (containing 950 mosquitoes) were WNV-positive and 8 of 28 (28.6%) Cx. tarsalis pools (containing 486 mosquitoes) were WNV-positive. Positive mosquito pools were detected at 9 of the 20 trap sites (Fig. 1). Efforts to evaluate dependence of infection rate on distance to nearest communal roosts were unsuccessful due to insufficient data for robust or reliable estimation, despite aggregation across Culex species and over the period of the study (Fig. 2). Relatively few mosquitoes were collected at several trap locations, as evidenced by the wide infection rate CIs.
Discussion
A study of avian hosts of WNV in the East Valley of metropolitan Phoenix, Arizona, found elevated seroprevalence (i.e., above 50%) in several species of communally roosting birds following the human WNV epidemic that occurred there June–August, 2010 (Komar et al. 2013). In particular, house sparrow and great-tailed grackle were found to be roosting abundantly in a suburban area where numerous human cases had occurred. In this paper, we mapped these communal roosts and tested them for a spatial association with human WNV cases and WNV-infected mosquitoes. A potential limitation in the study is the collection of roost location data late in the epidemic period. Ideally these data would have been collected in July at the peak of human exposure, in case of potential shifts in roost locations. Although we mapped the location of the communal roost sites at the end of the outbreak period in September, we expect that non-breeding individual birds use these sites year-round, and that juvenile birds produced throughout the spring and early summer join the sites shortly after fledging. In the case of the great-tailed grackle, the majority of the annual crop of juveniles would be using the sites by mid-June, when WNV transmission to humans was reaching epidemic levels (Corman and Wise-Gervais 2005).
Published studies of WNV transmission risk at communal roosts of ardeids (herons and egrets) and American robin have reported mixed findings, with some detecting higher transmission risk near the communal roost sites (Reisen et al. 2009; Diuk-Wasser et al. 2010) and others not (Reisen et al. 2005; Benson et al. 2012).Given the recent occurrence of an outbreak of human WNV cases in Phoenix, we expected to find a positive spatial association between avian communal roosting and WNV transmission in our study site. Instead, we found a negative association of communal bird (great-tailed grackle) roost sites with respect to residences of WNV-infected people.
Two possible explanations for this unexpected observation for great-tailed grackle communal roost sites (i.e., that they are negatively associated with transmission) are that (1) grackle roosts are located in habitats unsuitable for Culex host-seeking behavior and (2) grackle roosts protect against WNV infection of people. In the first scenario, the great-tailed grackle may select communal roost sites in locations where host-seeking mosquitoes are scarce, thus avoiding a positive geographic association with human WNV infections. This selection may reflect purposeful avoidance of mosquito attack, or may be driven by other factors which indirectly result in lower exposure to biting mosquitoes. In the second scenario, several theories could explain a protective effect of communal grackle roosts for WNV transmission to people (Table 2). First, the great-tailed grackle may repel Culex mosquitoes. Second, the great-tailed grackle may attract Culex mosquitoes but because of incompetence for infecting mosquitoes, cause a net zooprophylaxis for human infection with WNV. Third, feeding on grackles may be hazardous to mosquito survival, presumably through grackle defensive behaviors such as consumption of host-seeking mosquitoes. Fourth, the high density of birds within a communal grackle roost may reduce the local vector-host ratio and therefore lower virus amplification potential within the roost site (Janousek et al. 2014). Lastly, the additional species that often roost together with grackles (such as doves, starlings, and other blackbird species) may dilute the force of transmission within a grackle roost compared to single-species communal roosts like those of the house sparrow.
In regard to the first theory, which we term the Vector Repulsion Theory, feeding studies of both Cx. quinquefasciatus and Cx. tarsalis have observed that blood meals from grackles and other icterids are detected with lower frequency than expected from the relative abundance of these birds within the broader avian community, suggesting reduced host selection of icterids by these mosquitoes (Kent et al. 2009; Thiemann et al. 2012; Kading et al. 2013). However, whether this is due to reduced host preference or to low survival of mosquitoes that select icterids for blood meals is difficult to determine. If it is due to low preference by vectors, then anti-WNV antibody prevalence in icterids would also be lower relative to preferred species such as mourning dove and American robin, which has indeed been observed in some studies (Komar et al. 2005; Loss et al. 2009). However, other serosurveys have observed high exposure levels in grackles relative to other species (Godsey et al. 2005; Komar et al. 2012, 2013; Morales-Betoulle et al. 2013). The great-tailed grackle population sampled in our study site was found to have a WNV seroprevalence of approximately 86% (Komar et al. 2013).This observed high seroprevalence contradicts the theory that grackles repel Culex mosquitoes.
In regard to the second theory, which we term the Zooprophylaxis Theory, the grackle would be incompetent to transmit WNV infection to mosquitoes. However, data from experimental infections demonstrate that the great-tailed grackle is an efficient transmitter of WNV to Culex mosquitoes, and therefore highly competent (Guerrero-Sánchez et al. 2011). Nonetheless, immune individuals would be incompetent. Thus, the WNV-amplification potential of the great-tailed grackle in a communal roost depends inversely on the proportion of the birds that already circulate anti-WNV antibodies. Our observation that the communal grackle roosts are negatively associated with WNV transmission that had occurred earlier in the year (i.e., June–July, the period when most human infections occurred) suggests that immunity rates among the grackles may have already been high at that time. The high seroprevalence observed in the local grackle population supports this theory of zooprophylaxis due to immune-mediated incompetence.
The third theory, which we term the Vector Mortality Theory, derives from the concept that blood-feeding is hazardous to a mosquito’s survival. This has been studied for other species of birds, but not great-tailed grackle. Vertebrate hosts utilize numerous defensive behaviors to repel host-seeking mosquitoes, some of which result in mosquito death (Spielman and Edman 1988). A videotaped study of defensive behaviors of roosting American robin (Turdus migratorius) and European starling (Sturnus vulgaris) confirmed that both passerine species occasionally snatched host-seeking mosquitoes with their beaks and swallowed them (Hodgson 1998). This behavior was detected at dusk and in darkness using infrared video. The rate of mosquito capture was greater in daylight. The great-tailed grackle frequently roosts near artificial lights in urban settings (author’s personal observation), which could aid in this form of mosquito defense. Note that grackles may still become infected with WNV either from probing by infectious mosquitoes prior to being dispatched by the grackles’ defensive behaviors, or by per os consumption of infected mosquitoes. Consumption of infected mosquitoes likely results in productive infections in the grackle, as has been shown experimentally for house finch (Komar et al. 2003). Also, interrupted feeding due to anti-mosquito defensive behaviors has been shown to increase vector-host contacts (Hodgson et al. 2001).Thus, the high seroprevalence in the local grackle population is not inconsistent with this theory that the grackle may kill many host-seeking Culex mosquitoes. Furthermore, two blood meal host identification studies in the study site in August and September detected just four Culex blood meals (n = 190) that were derived from great-tailed grackle, inferring a low rate of repletion for Culex mosquitoes feeding on grackles in the locations where these mosquitoes were collected (Godsey et al. 2012; Komar et al. 2013).
The fourth theory, which we term Density-dependent Dilution, stems from the observation that grackles joining the communal roosts perch close together relative to other communally roosting species such as egrets, robins, and crows. Theoretically, this high density of hosts reduces the vector-host ratio and ostensibly makes it more difficult for an infectious mosquito to make contact with a susceptible amplifier, or for an uninfected vector mosquito to make contact with an infectious bird (Janousek et al. 2014). Mathematical modeling has demonstrated that reducing the vector-host ratio effectively reduces the force of the epidemic wave for WNV, which would result in fewer infected mosquitoes and lower transmission risk to humans (Magori et al. 2011).
The fifth theory, which we term Biodiversity-dependent Dilution, stems from the qualitative observation that the grackle often joins other species in mixed-species communal roosts. If some of these species are immune or otherwise incompetent to amplify WNV, then they may serve as a distraction for Culex vectors to feed on the WNV-amplifying grackle, and thereby reduce the force of transmission at the communal roost site. European starling, red-winged blackbird, and doves of various species are low-competence species that were variously present in the grackle roosts in Phoenix (Komar et al. 2003). The theory that increased biodiversity tends to have a diluting effect on the force of transmission has been demonstrated for WNV (Ezenwa et al. 2006).
In contrast to the negative association of communal grackle roosts with WNV illness in humans, there was insufficient evidence to conclude an association between communal house sparrow roosts and human WNV illness (P > 0.05). The house sparrow has been implicated as an important amplifier of WNV in Phoenix (Komar et al. 2013) and other outbreak locations, such as Florida, Louisiana, California, and Puerto Rico (Loss et al. 2009; Molaei et al. 2010; Komar et al. 2012). A communal house sparrow roost in Colorado was implicated as the source of a several WNV-infected Culex spp. mosquitoes which were co-located with the sparrow roost site, and some of which had recently fed on house sparrow blood (Kent et al. 2009). The reason why the house sparrow may enhance WNV transmission is that immunity levels in the sparrow population rarely achieve herd immunity (unpublished data), due in part to rapid population turnover (Lowther and Cink 2006).
The geographic pattern of human WNV cases in our study site could be explained by this hypothetical scenario: communal roosts of house sparrow and other competent species (such as house finch) amplify the virus through July until immunity builds up to levels sufficient for quenching WNV transmission to humans, beginning in August when the outbreak subsides. However, transmission-quenching by grackle roosts begins earlier, thus protecting certain neighborhoods during the peak of transmission.
One limitation of our study is the small number of communal grackle roosts present in the 40-square-mile study area. To provide reliable inference in light of small sample sizes and potential skewness in the observations, we used exact permutation methods to compare the mean and median distances between case and control locations. Nevertheless, ecological relationships, such as any association between communal house sparrow roost sites and WNV transmission not detected here, are already difficult to detect due to the chaotic nature of ecology. The environment is not static, and many environmental conditions present challenges for accurate detection of ecological associations. For example, the geographic locations of WNV case residences are approximations of where each human WNV infection actually took place, and presumably a significant source of error in our risk assessment. The set of communal roost locations is probably a subset of the true number of roost locations within the study site, as it is possible that not all were detected. Finally, the roost locations may have shifted in the weeks between the peak of epidemic transmission in July and the roost-mapping effort in September. While major roost sites tend to be conserved over a period of many years, roost sites with smaller numbers of birds often are ephemeral, as the birds later join larger roosts. The shifting nature of these bird populations may lead to further interference with inference.
Conclusion
In conclusion, whereas the role of communal house sparrow roosts in amplifying WNV was ambiguous in the southeast section of Maricopa County, communal great-tailed grackle roost sites were negatively associated with residence locations of human victims of WNV. We cannot rule out that the locations of sparrow and grackle communal roost sites may have shifted in the time interval between the peak of outbreak activity (July) and the period of roost data collection (September), which could have biased the results obtained in this study. Furthermore, our conclusion may or may not be generalizable to other WNV transmission foci. However, the data support the observation that people living in proximity to great-tailed grackle communal roosts in our study area were less likely to have been infected with WNV during the outbreak of 2010. The complex interactions between WNV, its Culex vectors, and its vertebrate hosts require more study to understand the factors leading to virus amplification and quenching in the community, and the complex role that communal bird roosts may play in this transmission.
References
Benson TJ, Ward MP, Lampman RL, Raim A, Weatherhead PJ.(2012) Implications of spatial patterns of roosting and movements of American robins for West Nile virus transmission. Vector Borne Zoonotic Dis 12:877–885. DOI: 10.1089/vbz.2011.0902 [Online May 31, 2012]
Biggerstaff BJ.(2006) PooledInfRate, Version 3.0: A Microsoft ® Excel ® Add-in to Compute Prevalence Estimates from Pooled Samples, Fort Collins, USA: Centers for Disease Control and Prevention
Canty A, Ripley B (2013) Boot: Bootstrap R (S-plus) functions. In: R package version 1.3-9
Corman T, Wise-Gervais C (2005) Arizona Breeding Bird Atlas, Albuquerque: University of New Mexico Press
Davison AC,Hinkley DV (1997) Bootstrap Methods and Their Applications, Cambridge: Cambridge University Press
Diuk-Wasser MA, Molaei G, Simpson JE, Folsom-O’Keefe CM, Armstrong PM, Andreadis TG (2010) Avian communal roosts as amplification foci for West Nile virus in urban areas in northeastern United States. Am J Trop Med Hyg 82:337–343. DOI: 10.4269/ajtmh.2010.09-0506
Ezenwa VO, Godsey MS, King RJ, Guptill SC (2006)Avian diversity and West Nile virus: testing associations between biodiversity and infectious disease risk.ProcBiolSci 273:109–117
Gibney KB, Colborn J, Baty S, Bunko Patterson AM, Sylvester T, Briggs G, et al. (2012) Modifiable risk factors for West Nile virus infection during an outbreak–Arizona, 2010. Am J Trop Med Hyg 86:895–901. DOI: 10.4269/ajtmh.2012.11-0502
Godsey MS Jr, Blackmore MS, Panella NA, Burkhalter K,Gottfried K, Halsey LA, et al. (2005) West Nile virus epizootiology in the southeastern United States, 2001.Vector Borne Zoonotic Dis 5:82–89
Godsey MS Jr, Burkhalter K, Young G, Delorey M, Smith K, Townsend J, et al. (2012) Entomologic investigations during an outbreak of West Nile virusdisease in Maricopa County, Arizona, 2010. Am J Trop Med Hyg 87:1125-1131. DOI: 10.4269/ajtmh.2012.11-0700
Guerrero-Sánchez S, Cuevas-Romero S, Nemeth NM, Trujillo-Olivera MT, Worwa G, Dupuis A, et al.(2011) West Nile virus infection of birds, Mexico.Emerg Infect Dis 17:2245-2252. DOI:10.3201/eid1712.110294
Hayes EB, Komar N, Nasci RS, Montgomery SP, O’Leary DR, Campbell GL (2005) Epidemiology and transmission dynamics of West Nile virus disease.Emerg Infect Dis 11:1167-1173
Hepworth G (2005) Confidence intervals for proportions estimated by group testing with groups of unequal size.J AgricBiol Environ Stat10:478–497
Hodgson JC (1998) European starlings (Sturnus vulgaris) and American robins (Turdus migratorius) as hosts for the eastern equine encephalitis vector mosquito, Culiseta melanura (Diptera: Culicidae). Master of Science Thesis, Boston: University of Massachusetts, pp 40–41
Hodgson JC, Spielman A, Komar N, Krahforst CF, Wallace GT, Pollack RJ (2001) Interrupted blood-feeding by Culiseta melanura (Diptera: Culicidae) on European starlings. J Med Entomol38:59-66
Janousek WM, Marra PP, Kilpatrick AM (2014) Avian roosting behavior influences vector-host interactions for West Nile virus hosts. Parasites and Vectors 7:399. DOI: 10.1186/1756-3305-7-399, http://www.parasitesandvectors.com/content/7/1/399; Accessed Oct 14, 2014
Kading RC, Reiche AS, Morales-Betoulle ME, Komar N (2013) Host selection of potential West Nile virus vectors in Puerto Barrios, Guatemala, 2007. Am J Trop Med Hyg 88:108-115. DOI: 10.4269/ajtmh.2012.12-0223
Kent R, Juliusson L, Weissmann M, Evans S, Komar N (2009) Seasonal blood-feeding behavior of Culex tarsalis (Diptera: Culicidae) in Weld County, Colorado, 2007. J Med Entomol 46:380-390
Kilpatrick AM (2011) Globalization, land use, and the invasion of West Nile virus.Science 334:323–327. DOI: 10.1126/science.1201010
Komar N, Bessoff K, Diaz A, Amador M, Young G, Seda R, et al.(2012) Avian hosts of West Nile virus in Puerto Rico. Vector Borne Zoonotic Dis 12:47-54. DOI:10.1089/vbz.2011.0609
Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, et al. (2003) Experimental infection of North American birds with the New York 1999 strain of West Nile virus.Emerg Infect Dis 9:311-322
Komar N, Panella NA, Langevin SA, Brault AC, Amador M, Edwards E, Owen JC (2005) Avian hosts for West Nile virus in St. Tammany Parish, Louisiana, 2002. Am J Trop Med Hyg 73:1031-1037
Komar N, Panella NA, Young GR, Brault AC, Levy CE (2013) Avian hosts of West Nile virus in Arizona. Am J Trop Med Hyg 89:474-481. DOI:10.4269/ajtmh.13-0061
Lehmann EL (1991)Testing Statistical Hypotheses, 2nd Ed,Belmont CA: Wadsworth Inc.
Loss SR, Hamer GL, Walker ED, Ruiz MO, Goldberg TL, Kitron UD, Brawn JD (2009) Avian host community structure and prevalence of West Nile virus in Chicago, Illinois.Oecologia 159:415-424. DOI: 10.1007/s00442-008-1224-6
Lowther PE, Cink CL (2006) House sparrow (Passer domesticus). In: The birds of North America online, Poole A (Editor), Ithaca: Cornell Laboratory of Ornithology. http://bna.birds.cornell.edu/bna/species/012. Accessed November 27, 2012
Magori K, Bajwa WI, Bowden S, Drake JM (2011) Decelerating spread of West Nile virus by percolation in a heterogeneous urban landscape. PLOS Computational Biology 7:e1002104. DOI: 10.1371/journal.pcbi.1002104
Molaei G, Cummings RF, Su T, Armstrong PM, Williams GA, Cheng ML, et al. (2010) Vector-host interactions governing epidemiology of West Nile virus in Southern California. Am J Trop Med Hyg 83:1269-1282. DOI:10.4269/ajtmh.2010.10-0392
Morales-Betoulle ME, Komar N, Panella NA, Alvarez D, López MR, BetoulleJL,et al. (2013) West Nile virus ecology in a tropical ecosystem in Guatemala. Am J Trop Med Hyg 88:116-126. DOI:10.4269/ajtmh.2012.12-0276
PazS,Semenza JC (2013) Environmental drivers of West Nile fever epidemiology inEurope and Western Asia–a review. Int J Environ Res Public Health 10: 3543–3562. DOI: 10.3390/ijerph10083543
Reisen WK, Wheeler S, Armijos MV, Fang Y, Garcia S, Kelley K, Wright S (2009) Role of communally nesting ardeid birds in the epidemiology of West Nile virusrevisited. Vector Borne Zoonotic Dis 9:275-280. DOI:10.1089/vbz.2008.0104
Reisen WK, Wheeler SS, Yamamoto S, Fang Y, Garcia S (2005) Nesting Ardeid colonies are not a focus of elevated West Nile virus activity in southern California. Vector Borne Zoonotic Dis 5:258-266
Spielman A,Edman JD (1988) Blood-feeding by vectors: physiology, ecology, behavior, and vertebrate defense. In: The Arboviruses: Volume 1, Monath TP (editor), Boca Raton: CRC Press, pp 153–189
Thiemann TC, Lemenager DA, Kluh S, Carroll BD, Lothrop HD, Reisen WK (2012) Spatial variation in host feeding patterns of Culex tarsalis and the Culex pipiens complex (Diptera: Culicidae) in California. J Med Entomol 49:903-916
Ward MP, Raim A, Yaremych-Hamer S, Lampman R, Novak RJ (2006) Does the roosting behavior of birds affect transmission dynamics of West Nile virus? Am J Trop Med Hyg 75:350–355
Xi M (2001) Regression analysis of group testing samples.Stat Med20: 1957–1969
Acknowledgments
We thank the Maricopa County Environmental Services Department Vector Control Division and the Maricopa County Department of Public Health staff for generating the mosquito and human surveillance data used for this study. Funding was provided by the Centers for Disease Control and Prevention. The authors declare no conflict of interest. The statements and opinions expressed in this article are those of the authors and do not necessarily represent official policy of the institutions with which the authors are affiliated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Komar, N., Colborn, J.M., Horiuchi, K. et al. Reduced West Nile Virus Transmission Around Communal Roosts of Great-Tailed Grackle (Quiscalus mexicanus). EcoHealth 12, 144–151 (2015). https://doi.org/10.1007/s10393-014-0993-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-014-0993-0