Abstract
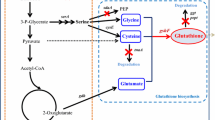
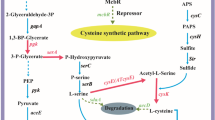
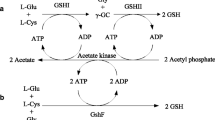
Glutathione (GSH) is an important bioactive substance applied widely in pharmaceutical and food industries. Due to the strong product inhibition in the GSH biosynthetic pathway, high levels of intracellular content, yield and productivity of GSH are difficult to achieve. Recently, a novel bifunctional GSH synthetase was identified to be less sensitive to GSH. A recombinant Escherichia coli strain expressing gshF encoding the bifunctional glutathione synthetase of Streptococcus thermophilus was constructed for GSH production. In this study, efficient GSH production using this engineered strain was investigated. The cultivation process was optimized by controlling dissolved oxygen (DO), amino acid addition and glucose feeding. 36.8 mM (11.3 g/L) GSH were formed at a productivity of 2.06 mM/h when the amino acid precursors (75 mM each) were added and glucose was supplied as the sole carbon and energy source.





Similar content being viewed by others
References
Alfafara CG, Kanda A, Shioi T, Shimizu H, Shioya S, Suga K (1992) Effect of amino acids on glutathione production by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 36:538–840
Awano N, Wada M, Kohdoh A, Oikawa T, Takagi H, Nakamori S (2003) Effect of cysteine desulfhydrase gene disruption on l-cysteine overproduction in Escherichia coli. Appl Microbiol Biotech 62:239–243
Eiteman MA, Altman E (2006) Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends Biotechnol 24(11):530–536
Ge SL, Zhu TC, Li Y (2012) Expression of bacterial GshF in Pichia pastoris for glutathione production. Appl Environ Microbiol 78:5435–5439
Gopal S, Borovok I, Ofer A, Yanku M, Cohen G, Goebel W, Kreft J, Aharonowitz Y (2005) A multidomain fusion protein in Listeria monocytogenes catalyzes the two primary activities for glutathione biosynthesis. J Bacteriol 187:3839–3847
Gushima H, Miya T, Murata K, Kimura A (1983) Construction of glutathione-producing strains of Escherichia coli B by recombinant DNA techniques. J Appl Biochem 5:43–52
Hibi T, Nii H, Nakatsu T, Kimura A, Kato H, Hiratake J, Oda J (2004) Crystal structure of gamma-glutamylcysteine synthetase: insights into the mechanism of catalysis by a key enzyme for glutathione homeostasis. Proc Natl Acad Sci USA 101:15052–15057
Janowiak BE, Griffith OW (2005) Glutathione synthesis in Streptococcus agalactiae. One protein accounts for gamma-glutamylcysteine synthetase and glutathione synthetase activities. J Biol Chem 280:11829–11839
Johnston WA, Stewart M, Lee P, Cooney MJ (2003) Tracking the acetate threshold using DO-transient control during medium and high cell density cultivation of recombinant Escherichia coli in complex media. Biotechnol Bioeng 84:314–323
Kono G, Harada M, Sugisaki K, Nishida M (1977) High glutathione-containing yeast. JP patent 52(125):687
Li W, Li ZM, Yang JH, Ye Q (2011) Production of glutathione using a bifunctional enzyme encoded by gshF from Streptococcus thermophilus expressed in Escherichia coli. J Biotechnol 154:261–268
Li Y, Chen J, Mao YY, Lun SY, Koo YM (1998) Effect of additives and fed-batch culture strategies on the production of glutathione by recombinant Escherichia coli. Process Biochem 33:709–714
Li Y, Wei GY, Chen J (2004) Glutathione: a review on biotechnological production. Appl Microbiol Biotechnol 66:233–242
Liang GB, Du GC, Chen J (2009) Salt-induced osmotic stress for glutathione overproduction in Candida utilis. Enzym Microb Technol 45:324–329
Liao XY, Shen W, Chen J, Li Y, Du GC (2006) Improved glutathione production by gene expression in Escherichia coli. Lett Appl Microbiol 43:211–214
Lin J, Liao XY, Du GC, Chen J (2009) Enhancement of glutathione production in a coupled system of adenosine deaminase-deficient recombinant Escherichia coli and Saccharomyces cerevisiae. Enzym Microb Technol 44:269–273
Lin J, Liao XY, Zhang J, Du GC, Chen J (2009) Enhancement of glutathione production with a tripeptidase-deficient recombinant Escherichia coli. J Ind Microbiol Biotechnol 36(12):1447–1452
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760
Murata K, Kimura A (1982) Cloning of a gene responsible for the biosynthesis of glutathione in Escherichia coli B. Appl Environ Microbiol 44:1444–1448
Murata K, Tani K, Kato J, Chibata I (1981) Glycolytic pathway as an ATP generation system and its application to the production of glutathione and NADP. Enzym Microb Technol 3:233–242
Nie W, Wei GY, Du GC, Li Y, Chen J (2005) Enhanced intracellular glutathione synthesis and excretion capability of Candida utilis by using a low pH-stress strategy. Lett Appl Microbiol 40:378–384
Shiloach J, Fass R (2005) Growing E. coli to high cell density—A historical perspective on method development. Biotechnol Adv 23:345–357
Shimizu H, Araki K, Shioya S, Suga K (1991) Optimal production of glutathione by controlling the specific growth rate of yeast in fed-batch culture. Biotechnol Bioeng 38:196–205
Zawada J, Swartz J (2005) Maintaining rapid growth in moderate-density Escherichia coli fermentations. Biotechnol Bioeng 89:407–415
Acknowledgments
This study was supported by the National Science Foundation for Young Scientist of China (Grant No. 21406065), the Fundamental Research Funds for the Central Universities (Grant No. 222201313007 & 22A201514042), the National Special Fund for State Key Laboratory of Bioreactor Engineering (No. 2060204) and Shanghai Committee of Science and Technology (Grant No. 13DZ1930202).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, D., Wang, C., Wu, H. et al. Glutathione production by recombinant Escherichia coli expressing bifunctional glutathione synthetase. J Ind Microbiol Biotechnol 43, 45–53 (2016). https://doi.org/10.1007/s10295-015-1707-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-015-1707-5