Abstract
Multiple sclerosis (MS) is a chronic, inflammatory, autoimmune disease of the central nervous system, and is an important cause of disability in young adults. In genetically susceptible individuals, several environmental factors may play a partial role in the pathogenesis of MS. Some studies suggests that high-salt diet (>5 g/day) may contribute to the MS and other autoimmune disease development through the induction of pathogenic Th17 cells and pro-inflammatory cytokines in both humans and mice. However, the precise mechanisms of pro-inflammatory effect of sodium chloride intake are not yet explained. The purpose of this review was to discuss the present state of knowledge on the potential role of environmental and dietary factors, particularly sodium chloride on the development and course of MS.
Similar content being viewed by others
Introduction
Multiple sclerosis (MS) is a chronic, inflammatory, autoimmune disease of the central nervous system (CNS), and is a major cause of disability in young adults [1]. In genetically susceptible individuals, environmental factors play a significant partial role in the pathogenesis of MS [2]. Numerous studies examined the influence of environmental factors, such as Epstein–Barr infection [3–5], vitamin D levels [6–11], smoking [12, 13], obesity [14], sunlight exposure [15, 16], and geographic variation, on MS [17, 18].
Methodology
All quotable references were searched using Pubmed and Google scholar between March and May 2016. References that allowed free access to full text by the Medical University of Silesia were retrieved and read. The oldest publications were retrieved from the Medical University of Silesia Library. One reviewer performed literature searches and two other researchers independently screened the articles. All disagreements were discussed and resolved by the authors or in consultation with other experts. Information used in our review was evaluated using evidence-based medicine. Inclusion criteria for the articles were: original papers, systematic reviews, systematic summaries, and meta-analysis. We excluded publications written in foreign languages, such as Spanish, German, or Russian. Keywords used for literature searches were as follows: “multiple sclerosis”, “MS”, “sodium”, “sodium chloride”, “autoimmunity”, “diet”, and “VGSCs”. All keywords were in accordance with the MeSH terms. To identify the appropriate publications, we searched Pubmed and Google scholar using combinations of keywords in the following order: “MS and diet”, “MS and salt intake”, “MS and environmental factors,” and “MS and VGSCs”. After reading titles and abstracts, some articles from each combination of keywords were excluded. Most of the excluded articles were in a foreign language. The comprehensive literature search identified 907 articles that were relevant for our review. Titles, abstracts, or full articles were reviewed to determine whether each search result matched our selection criteria. We also reviewed the references of the selected original papers and review articles found by our search for additional papers relevant to our review. Only high-quality publications from the last decades were included. In addition, 306 articles were retrieved from the Medical University of Silesia Library. Of these 306 articles, we eliminated those that were too general in scope for our review.
Environmental factors influencing MS
Although some studies confirmed the link between a previous infection with Epstein–Barr virus (EBV) and the development of multiple sclerosis [3–5, 19–21], the involvement of EBV in the etiology of MS is unclear. However, certain viral infections likely increase susceptibility to MS [22]. Sero-epidemiological studies have demonstrated that almost 100% of adult MS patients are infected with EBV [19]. Late childhood infection of EBV is proposed to be the serious risk factor for the disease. Moreover, there is a strong EBV-specific CD8+ response in the blood of MS patients in the beginning of the disease and the intensity decreases in the course of the illness [2–5, 19, 20].
Low serum vitamin D levels are currently one of the most studied environmental factors influencing the development of MS. It has been shown that intake of food rich in vitamin D significantly prevents the development of MS or reduce activity of the disease [6–9, 16]. Nevertheless, some authors did not report a protective role of vitamin D supplementation for the development of MS [9, 10].
Smoking is another possible factor for the development of MS or might be responsible for worst prognosis of course of the disease [13, 23]. Furthermore, smoking cessation improved the prognosis in patients with MS. Ramanujam et al. confirmed that the time to conversion to secondary progressive MS (SPMS) decreases by 4.7% for each additional year of smoking after the diagnosis in patients with RRMS (acceleration factor 1.047; 95% CI 1.023–1.072; P < 0.01) [13].
Obesity is a probable susceptibility factor for MS and several other autoimmune diseases [24], but the relationship between increased body mass index (BMI) and disease activity has not been fully explained. There is no doubt that obesity increases levels of pro-inflammatory cytokines and is associated with low-grade inflammatory state [25]. There are reports providing that RRMS activity is higher in obese and overweight patients than in patients with normal BMI undergoing IFN β treatment [14]. Moreover, Oliveira et al. reported a positive relationship between elevated BMI and disability in MS patients [26].
Ultraviolet radiation was proposed to be a significant environmental factor influencing prevalence of the disease [15]. Reduced risk of MS through exposure to sunlight is probably mediated not only by increased production of vitamin D in the skin, but also by the synthesis of anti-inflammatory factors, such as IL-10, TNF-α, and Treg cells [16]. Therefore, the latitude is nowadays considered to be related with prevalence of MS. The disease is less frequent near the equator and more frequent in northern countries [18, 27]. Exceptions to this trend, namely, Sardinia, where the prevalence of the disease is significantly higher [28], and northern Scandinavia, with markedly low prevalence [29], may be due to genetic and behavioral factors [18].
Influence of diet on MS
It seems that diet might have a significant relationship with the inflammatory process of MS. Many studies have shown that diet plays the role in the pathogenesis of MS [17, 30–32]. Recent studies have provided the evidence for a protective role of polyunsaturated fatty acids on the risk of MS; however, there is no conclusive evidence for a beneficial role of polyunsaturated fatty acid supplementation in patients with MS. Hoare et al. demonstrated that the amount of omega-3 polyunsaturated fatty acids taken orally is inversely proportional to the risk of demyelination in the CNS [33]. Moreover, Khalili et al. found a strong correlation between oral intake of lipoic acid (1.2 mg/day) and decrease in the levels of pro-inflammatory cytokines, including INF-γ, ICAM-1, and anti-inflammatory cytokines, including TGF-β and IL-4, compared with placebo group [35]. On the other hand, Torkildsen et al. showed that consumption of omega-3 fatty acids used as monotherapy or in combination with interferon beta-1a had no beneficial effect on the disease compared to placebo [34]. Retinoic acid (RA), an active metabolite of vitamin A, revealed a strong immunosuppressive activity [36]. RA has been shown to modulate the balance between Th1/Th2 and Th17/Treg cells and B cell function, contributing to augmented tolerance and inhibited inflammatory response. It also contributes to enhanced tolerance and reduction of inflammatory effects [37]. Bitarafan et al. investigated the impact of vitamin A on disease progression in MS patients. The study evaluated the expanded disability status scale (EDSS) and MS functional composite (MSFC). The results showed that vitamin A improved MSFC in RRMS patients, but did not affect EDSS, relapse rate, or active brain lesions in MRI [38]. Ketogenic diet (high amount of fat, decreased protein content, and very low carbohydrates) was shown as potentially therapeutic in progressive forms of MS, which is especially relevant, because currently, there is no treatment for progressive forms of the disease [39, 40]. Kim et al. reported that ketogenic diet improved motor disability and cognitive impairment in mice with experimental autoimmune encephalomyelitis compared with mice on the standard diet. Furthermore, a ketogenic diet reversed structural brain lesions and reduced CNS inflammation and oxidative stress [41]. On the other hand, it was reported that creatine supplementation did not improve muscle capacity or habitual fatigue in MS individuals [42] or that restricted intake of animal fat (no more than 10–15 g/day) caused remission of the disease in patients with RRMS [43].
Polyphenols and carotenoids from vegetables, n-3 PUFA from fish, vitamins A, C, D, and E, thiol compounds, such as lipoic acid, and oligoelements, such as selenium and magnesium, have anti-oxidant properties [44, 45]. Th17 cells, which produce pro-inflammatory cytokines, are increased, whereas Treg cells are decreased in MS, and thus, the balance between Th17 and Treg cells is impaired in this disease. Vitamin A and its active metabolites (all-trans-retinoic acid and 9-cis-retinoic acid) modulate the imbalance of Th17 and Treg cells and might be beneficial to the prevention and treatment of MS [46]. Moreover, this vitamin was proposed to have a beneficial effect during interferon therapy and improved psychiatric outcomes for anti-inflammatory mechanisms [37].
Sodium channels in MS
Voltage-gated sodium channels (VGSCs) are key mediators of action potential initiation and propagation in excitable cells [47–49]. Their expression has also been reported in cell types that are traditionally regarded as non-excitable, including glia, human vascular endothelial cells, human epidermal keratinocytes, and carcinoma cells, where their role is less clear [50–55]. Aberrant functional expression/activity of VGSCs has been identified as a major contributing factor in a number of human pathologies, including cardiac arrhythmia [55], epilepsy [56, 57, 58], pain [59, 60], periodic paralysis [61, 62], migraine [63], MS [64], and cancer [65]. VGSCs exist as heteromeric membrane-bound protein complexes that typically consist of a single pore-forming α subunit in association with one or more β subunits [66, 67].
The mammalian sodium channels include ten members (Nav1.1–Nav1.9 and Nax) encoded by genes SCN1A–SCN11A. While substantial homology exists between the isoforms, differences in amino acid sequence confer distinct voltage dependence, kinetic and pharmacological properties on each of the isotypes [68, 69]. Data concerning the location and function of each VGSCs subunit are included in Table 1.
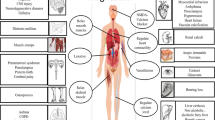
β subunits (β1–β4) combine in vivo with either β1 or β3 through non-covalent bonding and with either β2 or β4 via a covalent bond [101–104]. Numerous studies have revealed the presence of Nav1.1, Nav1.2, Nav1.3 [105], Nav1.6 [106], and Nav1.5 [107] in rodent astrocytes. Sodium channels in these glial cells are localized to the plasma membrane, where they mediate sodium currents [108]. The star-shaped glial cells situated in the CNS take an essential part in the response of the CNS to injury, including inflammation and degeneration in MS. Herzog et al. have shown that VGSCs can contribute to axonal injury in MS by providing a pathway for sustained sodium influx that drives the Na+/Ca2+ exchanger to import calcium into axons [109]. Elevated calcium levels can activate nitric oxide synthase and deleterious proteolytic enzymes [109–112]. The harmful effects of nitric oxide on mitochondrial function include a reduction in adenosine triphosphate (ATP) levels and an exhaustion of sodium–potassium adenosine triphosphatase (Na+K+-ATPase), hence compromising the axons’ capacity to maintain normal transmembrane sodium gradient. This action provides a positive feedback loop that imports even more intracellular calcium, thereby further enhancing the damage [113]. Consequently, these mechanisms lead to axonal injury and further to disability (Fig. 1).
Numerous studies have shown that partial blockade of voltage-gated sodium channels could result in neuroprotection in patients with MS [112]. Indeed, the axonal protection has been demonstrated in animals with experimental autoimmune encephalomyelitis (EAE) by means of the sodium channel blocking drugs flecainide [114, 115], safinamide [115], phenytoin [116], and, recently, lamotrigine [117]. Neuroprotection is emerging as a potentially important strategy for preventing disability progression in MS [118]. In contrast, some clinical studies do not support the protective role of VGSC blockers in MS. Counihan et al. studied 400 patients, 51 of whom received CBZ symptomatic therapy (average duration of therapy was 27 months), and showed that the long term exposure to the VGSC-blocking drug CBZ does not affect the long-term disability and disease progression in MS patients, despite studies in animals suggesting a neuroprotective role of VGSC blockers [119]. Furthermore, using CSF neurofilament (NfH) as a good surrogate marker of neurodegeneration in MS, Gnanapavan et al. revealed no benefit of lamotrigine in the prevention of axonal breakdown by lowering NfH levels compared to the placebo arm [120]. The protective role of lamotrigine is also disputed by Kapoor et al. [121], but it is premature to fully dismiss this hypothesis.
Sodium chloride intake and MS
High intake of sodium chloride is currently considered to be a potentially important factor influencing the onset of MS. Changes in eating habits that have occurred in recent decades in developed countries may account for an increase in incidence of MS and other autoimmune diseases [122]. Today’s typical Western diet includes more sodium chloride than it was in the past [123]. Therefore, popular processed meals, such as “fast food”, contain approximately 100 times more sodium chloride than homemade meals [124, 125]. Increased hypertonicity can stimulate the immune system [126], and furthermore, superior sodium chloride uptake can affect the innate immune system [127].
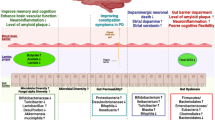
Recent studies have demonstrated the importance of interleukin-17 (IL-17)-induced CD4+ Th17 cell population in autoimmune diseases [128]. Kleinewietfield et al. showed that the addition of a modest amount of NaCl (40 mM) to a culture of differentiating Th17 cells caused a roughly logarithmic augmentation of IL-17A in naïve CD4 cells in vitro and this process was mediated by p38/MAPK, NFAT5, and SGK [122]. Moreover, high-salt concentration results in growth of pathogenic phenotype of Th17 cells [122, 129, 130]. Thus, the change in eating habits that includes a high amount of salt may contribute to the recent increase in MS incidence through the induction of pathogenic Th17 cells [122, 126, 128]. The Th17 cells induced by high-salt concentration upregulate the production of pro-inflammatory cytokines GM-CSF, TNFα, IL-2, IL-9, several chemokines [131, 132], and CCR6 [133], which are essential for the autoimmune function of Th17 cells. Higher Na+ concentration, such as that between 160 and 250 mM, in the interstitium and lymphoid tissue and significantly lower concentration of Na+ in plasma, approximately 140 mM, are likely to be the mechanism for decreasing the inflammatory response in the blood while favoring immune activation in lymphoid tissues or with migration of cells into tissue [127, 134]. Otherwise, diet rich in salt can affect the severity of the disease. Kleinewietfeld et al. showed that mice fed salty meals developed deterioration of EAE, with an increase in Th17 cell number and augmented infiltration of Th17 cells into the CNS [122]. Moreover, in an observational trial on 122 MS patients, Farez et al. demonstrated that the disease exacerbation rate was 2.75-fold in participants with medium salt intake (2–4.8 g/day) (95% CI 1.3–5.8) and 9.95-fold in participants with high sodium intake (4.8 g/day or more) (95% CI 1.4–11.2) compared with the low-intake group (under 2 g/day) [135]. This finding may be due to the fact that sodium concentration is tightly regulated within narrow limits regardless of large variations in sodium consumption, due to its importance in general metabolism [136]. The renin–angiotensin–aldosterone system (RAAS), which is a major regulator of blood pressure, also significantly affects autoimmunity in many diseases which include MS and its animal model—EAE. Han et al. showed that peptides related to the RAAS are present in CNS lesions of MS patients [137]. Sodium chloride, among many other physiological effects, modulates the renin–angiotensin system [138]. Interestingly, the activation of renin and angiotensin has been implicated in the pathogenesis of EAE [139]. Furthermore, increases in systolic blood pressure similar to those observed with high-salt consumption have recently been shown to be associated with the disruption of white matter integrity in young normotensive individuals [140]. In addition, Platten et al. demonstrated an increase in the expression of angiotensin receptor 1 (AT1R) in lymph node cells, indicating that AT1R is activated in antigen-specific T cells during the peripheral immune response to autoantigens. In addition, angiotensin II (AII) binding was augmented in Periodate–Lysine–Paraformaldehyde (PLP)-activated CD4 + T cells and to a lesser extent in activated CD11b + monocytes. Immunization with PLP139–151 led to an induction of AII in CD4 + T cells, CD11b + monocytes, and to an increase in serum AII levels, demonstrating that the RAAS is activated in peripheral immune cells. Pretreatment of mice immunized with PLP139–151 with the angiotensin converting enzyme (ACE) inhibitor lisinopril {N2-[(S)-1-carboxy-3-phenylpropyl]-L-lysyl-l-proline} or the AT1R antagonist candesartan (3-{[2′-(2 H -tetrazol-5-yl)biphenyl-4-yl]methyl}-2-ethoxy-3 H -benzo[d]imidazole-4-carboxylic acid) resulted in suppression of Th1 and Th17 cytokine release and up-regulation of immunosuppressive cytokines, such as IL-10 and transforming growth factor-β (TGF-β) [141]. Probable impact of high sodium diet on immune functions in MS patients was presented in Fig. 2.
Hucke et al. revealed a multidirectional activity of sodium chloride-rich diet in both humans and mice. Sodium chloride-rich diet promotes CNS autoimmunity, increases macrophage responses, skews the balance towards a pro-inflammatory M1 phenotype in macrophages, alters MAPK signaling in macrophages, and induces a pro-inflammatory phenotype in human monocytes [142]. In addition, Yi et al. demonstrated that high-salt intake promotes an increase in human serum monocytes, which play a pivotal role in the development of various immunological diseases [143]. Furthermore, Jörg et al. showed that a high-salt diet in the early phase of neuroinflammation mainly acts on Th17 cells and is independent of myeloid cells. This finding can help elucidate the impact of a high-salt diet on the emergence and course of autoimmune diseases [144]. Krementsov et al. demonstrated an increase in blood–brain barrier permeability and brain pathology in mice as a consequence of a high-salt diet, but did not demonstrate augmentation of Th17 or Th1 responses. Moreover, this study showed that the effects of dietary sodium on autoimmune neuroinflammation are sex-specific, genetically dependent, and CNS-mediated [145]. Furthermore, Zhou et al. demonstrated that a short-term increase in dietary salt intake could induce the expansion of CD14++CD16+ monocytes, as well as an increase in monocyte platelet aggregates (MPAs), which might be the cellular basis of high-salt-induced end organ inflammation and potential thromboembolic risk, independent of changes in blood pressure [146]. In addition, Hernandez et al. reported that excess dietary sodium intake lowers immunosuppressive actions of human and murine Foxp3+ Tregs in vitro and in vivo and is associated with increased Treg IFNγ secretion in vivo [147]. Data concerning the immunological effects of sodium chloride intake are shown in Table 2.
In conclusion, recent reports have demonstrated a potential pro-inflammatory role of excess sodium chloride intake in the pathogenesis of autoimmune and neurodegenerative diseases, both in vitro and in vivo, although the outcomes of these studies are not unanimous. Nevertheless, the current knowledge suggests that a low-salt diet (<5 g/day) might be beneficial in the prevention and treatment of autoimmune diseases, including MS.
References
Moghadasi AN, Pourmand S, Sharifian M et al (2016) Behavioral neurology of multiple sclerosis and autoimmune encephalopathies. Neurol Clin 34(1):17–31
Mandia D, Ferraro OE, Nosari G et al (2014) Environmental factors and multiple sclerosis severity: a descriptive study. Int J Environ Res Public Health 11(6):6417–6432
Ascherio A, Munger KL, Lennette ET et al (2001) Epstein-Barr virus antibodies and risk of multiple sclerosis: a prospective study. JAMA 286(24):3083–3088
Farrell RA, Antony D, Wall GR et al (2009) Humoral immune response to EBV in multiple sclerosis is associated with disease activity on MRI. Neurology 73(1):32–38
Ramroodi N, Niazi AA, Sanadgol N et al (2013) Evaluation of reactive Epstein-Barr Virus (EBV) in Iranian patient with different subtypes of multiple sclerosis (MS). Braz J Infect Dis 17(2):156–163
Pierrot-Deseilligny C (2009) Clinical implications of a possible role of vitamin D in multiple sclerosis. J Neurol 256(9):1468–1479
Cline J (2012) Calcium and vitamin d metabolism, deficiency, and excess. Top Companion Anim Med 27(4):159–164
Holmøy T, Torkildsen Ø, Myhr KM et al (2012) Vitamin D supplementation and monitoring in multiple sclerosis: who, when and wherefore. Acta Neurol Scand Suppl 195:63–69
Steffensen LH, Brustad M, Kampman MT (2013) What is needed to keep persons with multiple sclerosis vitamin D-sufficient throughout the year? J Neurol 260(1):182–188
Kampman MT, Steffensen LH, Mellgren SI et al (2012) Effect of vitamin D3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: exploratory outcomes from a double-blind randomised controlled trial. Mult Scler 18(8):1144–1151
Stein MS, Liu Y, Gray OM et al (2011) A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology 77(17):1611–1618
Hedström AK, Olsson T, Alfredsson L (2016) Smoking is a major preventable risk factor for multiple sclerosis. Mult Scler 22(8):1021–1026
Ramanujam R, Hedström AK, Manouchehrinia A et al (2015) Effect of smoking cessation on multiple sclerosis prognosis. JAMA Neurol 72(10):1117–1123
Kvistad SS, Myhr KM, Holmøy T et al (2015) Body mass index influence interferon-betatreatment response in multiple sclerosis. J Neuroimmunol 288:92–97
Sloka S, Silva C, Pryse-Phillips W et al (2011) A quantitative analysis of suspected environmental causes of MS. Can J Neurol Sci 38(1):98–105
Bäärnhielm M, Hedström AK, Kockum I et al (2012) Sunlight is associated with decreased multiple sclerosis risk: no interaction with human leukocyte antigen-DRB1*15. Eur J Neurol 19(7):955–962
Marrie RA (2004) Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol 3(12):709–718
Pugliatti M, Sotgiu S, Rosati G (2002) The worldwide prevalence of multiple sclerosis. Clin Neurol Neurosurg 104(3):182–191
Munger KL, Levin LI, O’Reilly EJ et al (2011) Anti-Epstein-Barr virus antibodies as serological markers of multiple sclerosis: a prospective study among United States military personnel. Mult Scler 17(10):1185–1193
Villoslada P, Juste C, Tintore M et al (2003) The immune response against herpesvirus is more prominent in the early stages of MS. Neurology 60(12):1944–1948
Yea C, Tellier R, Chong P et al (2013) Canadian pediatric demyelinating disease network. Epstein-Barr virus in oral shedding of children with multiple sclerosis. Neurology 81(16):1392–1399
Buljevac D, Flach HZ, Hop WC et al (2002) Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain 125(Pt5):952–960
Versini M, Jeandel PY, Rosenthal E et al (2014) Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev 13(9):981–1000
Cao H (2014) Adipocytokines in obesity and metabolic disease. J Endocrinol 220(2):T47–T59
Oliveira SR, Simão AN, Kallaur AP et al (2014) Disability in patients with multiple sclerosis: influence of insulin resistance, adiposity, and oxidative stress. Nutrition 30(3):268–273
Simpson S Jr, Blizzard L, Otahal P et al (2011) Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry 82(10):1132–1141
Harbo HF, Utsi E, Lorentzen AR et al (2007) Low frequency of the disease-associated DRB1*15-DQB1*06 haplotype may contribute to the low prevalence of multiple sclerosis in Sami. Tissue Antigens 69(4):299–304
Kingwell E, Marriott JJ, Jetté N et al (2013) Incidence and prevalence of multiple sclerosis in Europe: a systematic review. BMC Neurol 13:128
Kantarci O, Wingerchuk D (2006) Epidemiology and natural history of multiple sclerosis: new insights. Curr Opin Neurol 19(3):248–254
Coo H, Aronson KJ (2004) A systematic review of several potential non-genetic risk factors for multiple sclerosis. Neuroepidemiology 23(1–2):1–12
Smolders J, Damoiseaux J, Menheere P et al (2008) Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol 194(1–2):7–17
Pantzaris MC, Loukaides GN, Ntzani EE et al (2013) A novel oral nutraceutical formula of omega-3 and omega-6 fatty acids with vitamins (PLP10) in relapsing remitting multiple sclerosis: a randomised, double-blind, placebo-controlled proof-of-concept clinical trial. BMJ Open 3(4):e002170
Torkildsen O, Wergeland S, Bakke S et al (2012) ω-3 fatty acid treatment in multiple sclerosis (OFAMS Study): a randomized, double-blind, placebo-controlled trial. Arch Neurol 69(8):1044–1051
Khalili M, Azimi A, Izadi V et al (2014) Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation 21(6):291–296
Vergelli M, Olivotto J, Castigli E et al (1997) Immunosuppressive activity of 13-cis-retinoic acid in rats: aspects of pharmacokinetics and pharmacodynamics. Immunopharmacology 37(2–3):191–197
Hall JA, Grainger JR, Spencer SP et al (2011) The role of retinoic acid in tolerance and immunity. Immunity 35(1):13–22
Bitarafan S, Saboor-Yaraghi A, Sahraian MA et al (2016) Effect of vitamin A supplementation on fatigue and depression in multiple sclerosis patients: a double-blind placebo-controlled clinical trial. Iran J Allergy Asthma Immunol 15(1):13–19
Storoni M, Plant GT (2015) The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int 2015:681289
Allen BG, Bhatia SK, Anderson CM et al (2014) Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol 2:963–970
Kim do Y, Hao J, Liu R et al (2012) Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS One 7(5):e35476
Malin SK, Cotugna N, Fang CS (2008) Effect of creatine supplementation on muscle capacity in individuals with multiple sclerosis. J Diet Suppl 5(1):20–32
Swank RL, Goodwin J (2003) Review of MS patient survival on a Swank low saturated fat diet. Nutrition 19(2):161–162
Riccio P, Rossano R (2015) Nutrition facts in multiple sclerosis. ASN Neuro 7(1):1759091414568185. doi:10.1177/1759091414568185
Plemel JR, Juzwik CA, Benson CA et al (2015) Over-the-counter anti-oxidant therapies for use in multiple sclerosis: a systematic review. Mult Scler 21(12):1485–1495
Abdolahi M, Yavari P, Honarvar NM et al (2015) Molecular mechanisms of the action of vitamin A in Th17/Treg axis in multiple sclerosis. J Mol Neurosci 57(4):605–613
Bitarafan S, Saboor-Yaraghi A, Sahraian MA et al (2015) Impact of vitamin A supplementation on disease progression in patients with multiple sclerosis. Arch Iran Med 18(7):435–440
Hille B (1978) Ionic channels in excitable membranes. Current problems and biophysical approaches. Biophys J 22(2):283–294
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117:500–544
Barres BA, Chun LL, Corey DP (1990) Ion channels in vertebrate glia. Annu Rev Neurosci 13:441–474
Gautron S, Dos Santos G, Pinto-Henrique D et al (1992) The glial voltage-gated sodium channel: cell- and tissue-specific mRNA expression. Proc Natl Acad Sci USA 89(15):7272–7276
Gosling M, Harley SL, Turner RJ et al (1998) Human saphenous vein endothelial cells express a tetrodotoxin-resistant, voltage-gated sodium current. J Biol Chem 273(33):21084–21090
Diss JK, Fraser SP, Djamgoz MB (2004) Voltage-gated Na+ channels: multiplicity of expression, plasticity, functional implications and pathophysiological aspects. Eur Biophys J 33(3):180–193
Káradóttir R, Hamilton NB, Bakiri Y et al (2008) Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci 11(4):450–456
Zhao P, Barr TP, Hou Q et al (2008) Voltage-gated sodium channel expression in rat and human epidermal keratinocytes: evidence for a role in pain. Pain 139(1):90–105
Lopez-Santiago LF, Meadows LS, Ernst SJ et al (2007) Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J Mol Cell Cardiol 43:636–647
Wallace RH, Wang DW, Singh R et al (1998) Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet 19:366–370
Audenaert D, Claes L, Ceulemans B et al (2003) A deletion in SCN1B is associated with febrile seizures and early-onset absence epilepsy. Neurology 61:854–856
Scheffer IE, Harkin LA, Grinton BE et al (2007) Temporal lobe epilepsy and GEFS+ phenotypes associated with SCN1B mutations. Brain 130(Pt 1):100–109
Lopez-Santiago LF, Pertin M, Morisod X et al (2006) Sodium channel beta2 subunits regulate tetrodotoxin-sensitive sodium channels in small dorsal root ganglion neurons and modulate the response to pain. J Neurosci 26:7984–7994
Cummins TR, Sheets PL, Waxman SG (2007) The roles of sodium channels in nociception: implications for mechanisms of pain. Pain 131:243–257
Fontaine B, Khurana TS, Hoffman EP et al (1990) Hyperkalemic periodic paralysis and the adult muscle sodium channel alpha-subunit gene. Science 250:1000–1002
Waxman SG (2007) Channel, neuronal and clinical function in sodium channelopathies: from genotype to phenotype. Nat Neurosci 10:405–409
Dichgans M, Freilinger T, Eckstein G et al (2005) Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet 366:371–377
Waxman SG (2006) Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat Rev Neurosci 7:932–941
Fraser SP, Diss JK, Chioni AM et al (2005) Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res 11:5381–5389
Brackenbury WJ, Isom LL (2008) Voltage-gated Na+ channels: potential for beta subunits as therapeutic targets. Expert Opin Ther Targets 12(9):1191–1203
Brackenbury WJ, Djamgoz MB, Isom LL (2008) An emerging role for voltage-gated Na+ channels in cellular migration: regulation of central nervous system development and potentiation of invasive cancers. Neuroscientist 14(6):571–583
Roy ML, Narahashi T (1992) Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J Neurosci 12(6):2104–2111
Ikeda SR, Schofield GG, Weight FF (1986) Na+ and Ca2+ currents of acutely isolated adult rat nodose ganglion cells. J Neurophysiol 55(3):527–539
Sun LH, Yan ML, Hu XL et al (2015) MicroRNA-9 induces defective trafficking of Nav1.1 and Nav1.2 by targeting Navβ2 protein coding region in rat with chronic brain hypoperfusion. Mol Neurodegener 10:36
Ogiwara I, Miyamoto H, Morita N et al (2007) Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci 27(22):5903–5914
Catterall WA, Kalume F, Oakley JC (2010) NaV1.1 channels and epilepsy. J Physiol 588(Pt 11):1849–1859
Howell KB, McMahon JM, Carvill GL et al (2015) SCN2A encephalopathy: a major cause of epilepsy of infancy with migrating focal seizures. Neurology 85(11):958–966
Gazina EV, Leaw BT, Richards KL et al (2015) ‘Neonatal’ Nav1.2 reduces neuronal excitability and affects seizure susceptibility and behaviour. Hum Mol Genet 24(5):1457–1468
Dhalla AK, Yang M, Ning Y et al (2014) Blockade of Na+channels in pancreatic α-cells has antidiabetic effects. Diabetes 63(10):3545–3556
Lin CR, Chen KH, Yang CH et al (2014) Intrathecal miR-183 delivery suppresses mechanical allodynia in mononeuropathic rats. Eur J Neurosci 39(10):1682–1689
Tan AM, Samad OA, Dib-Hajj SD et al (2015) Virus-mediated knockdown of Nav1.3 in dorsal root ganglia of STZ-induced diabetic rats alleviates tactile allodynia. Mol Med 21:544–552
Zang Y, He XH, Xin WJ et al (2010) Inhibition of NF-kappaB prevents mechanical allodynia induced by spinal ventral root transection and suppresses the re-expression of Nav1.3 in DRG neurons in vivo and in vitro. Brain Res 1363:151–158
Bissay V, Van Malderen SC, Keymolen K et al (2016) SCN4A variants and Brugada syndrome: phenotypic and genotypic overlap between cardiac and skeletal muscle sodium channelopathies. Eur J Hum Genet 24(3):400–407
Cannon SC (2015) Channelopathies of skeletal muscle excitability. Compr Physiol 5(2):761–790
Nelson M, Yang M, Millican-Slater R et al (2015) Nav1.5 regulates breast tumor growth and metastatic dissemination in vivo. Oncotarget 6(32):32914–32929
Mohammed FH, Khajah MA, Yang M et al (2016) Blockade of voltage-gated sodium channels inhibits invasion of endocrine-resistant breast cancer cells. Int J Oncol 48(1):73–83
Musa H, Kline CF, Sturm AC et al (2015) SCN5A variant that blocks fibroblast growth factor homologous factor regulation causes human arrhythmia. Proc Natl Acad Sci USA 112(40):12528–12533
Tan BY, Yong RY, Barajas-Martinez H et al (2016) A Brugada syndrome proband with compound heterozygote SCN5A mutations identified from a Chinese family in Singapore. Europace 18(6):897–904
Andrikopoulos P, Fraser SP, Patterson L et al (2011) Angiogenic functions of voltage-gated Na+ channels in human endothelial cells: modulation of vascular endothelial growth factor (VEGF) signaling. J Biol Chem 286(19):16846–16860
O’Brien JE, Meisler MH (2013) Sodium channel SCN8A (Nav1.6): properties and de novo mutations in epileptic encephalopathy and intellectual disability. Front Genet 4:213
Hernandez-Plata E, Ortiz CS, Marquina-Castillo B et al (2012) Overexpression of NaV 1.6 channels is associated with the invasion capacity of human cervical cancer. Int J Cancer 130(9):2013–2023
Fertleman CR, Ferrie CD, Aicardi J et al (2007) Paroxysmal extreme pain disorder (previously familial rectal pain syndrome). Neurology 69(6):586–595
Suy S, Hansen TP, Auto HD et al. (2012) Expression of voltage-gated sodium channel Na(v)1.8 in human prostate cancer is associated with high histological grade. J Clin Exp Oncol. doi:10.4172/2324-9110.1000102
Han C, Huang J, Waxman SG (2016) Sodium channel Nav1.8: emerging links to human disease. Neurology 86(5):473–483
Roostaei T, Sadaghiani S, Park MT et al (2016) Channelopathy-related SCN10A gene variants predict cerebellar dysfunction in multiple sclerosis. Neurology 86(5):410–417
Woods CG, Babiker MO, Horrocks I et al (2015) The phenotype of congenital insensitivity to pain due to the NaV1.9 variant p. L811P. Eur J Hum Genet 23(10):1434
Leipold E, Hanson-Kahn A, Frick M et al (2015) Cold-aggravated pain in humans caused by a hyperactive NaV1.9 channel mutant. Nat Commun 6:10049
Xu W, Hong SJ, Zhong A et al (2015) Sodium channel Nax is a regulator in epithelial sodium homeostasis. Sci Transl Med 7(312):312ra177
Noda M, Hiyama TY (2015) Sodium sensing in the brain. Pflug Arch 467(3):465–474
Scheffer IE, Harkin LA, Grinton BE et al (2007) Temporal lobe epilepsy and GEFS+ phenotypes associated with SCN1B mutations. Brain 130(Pt 1):100–109
Watanabe H, Darbar D, Kaiser DW et al (2009) Mutations in sodium channel beta1- and beta2-subunits associated with atrial fibrillation. Circ Arrhythm Electrophysiol 2:268–275
Roger S, Rollin J, Barascu A et al (2007) Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int J Biochem Cell Biol 39:774–786
O’Malley HA, Shreiner AB, Chen GH et al (2009) Loss of Na+ channel beta2 subunits is neuroprotective in a mouse model of multiple sclerosis. Mol Cell Neurosci 40(2):143–155
Oyama F, Miyazaki H, Sakamoto N et al (2006) Sodium channel beta4 subunit: down-regulation and possible involvement in neuritic degeneration in Huntington’s disease transgenic mice. J Neurochem 98(2):518–529
Isom LL, De Jongh KS, Patton DE et al (1992) Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science 256(5058):839–842
Isom LL, Ragsdale DS, De Jongh KS et al (1995) Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell 83(3):433–442
Morgan K, Stevens EB, Shah B et al (2003) beta 3: an additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc Natl Acad Sci USA 97(5):2308–2313
Yu FH, Westenbroek RE, Silos-Santiago I et al (2003) Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J Neurosci 23(20):7577–7585
Black JA, Westenbroek R, Ransom BR et al (1994) Type II sodium channels in spinal cord astrocytes in situ: immunocytochemical observations. Glia 12:219–227
Reese KA, Caldwell JH (1999) Immunocytochemical localization of NaCh6 in cultured spinal cord astrocytes. Glia 26:92–96
Black JA, Dib-Hajj S, Cohen S et al (1998) Glial cells have heart: rH1 Na+ channel mRNA and protein in spinal cord astrocytes. Glia 23:200–208
Barres BA, Chun LL, Corey DP (1989) Glial and neuronal forms of the voltage-dependent sodium channel: characteristics and cell-type distribution. Neuron 2:1375–1388
Herzog RI, Liu C, Waxman SG et al (2003) Calmodulin binds to the C terminus of sodium channels Nav1.4 and Nav1.6 and differentially modulates their functional properties. J Neurosci 23:8261–8270
Kapoor R (2008) Sodium channel blockers and neuroprotection in multiple sclerosis using lamotrigine. J Neurolog Sci 274(1–2):54–56
Nikolaeva MA, Mukherjee B, Stys PK (2005) Na+-dependent sources of intra-axonal Ca2+ release in rat optic nerve during in vitro chemical ischemia. J Neurosci 25:9960–9967
Waxman SG (2008) Mechanisms of disease: sodium channels and neuroprotection in multiple sclerosis current status. Nat Clin Pract Neurol 4(3):159–169
Pappalardo LW, Samad OA, Black JA et al (2014) Voltage-gated sodium channel Nav 1.5 contributes to astrogliosis in an in vitro model of glial injury via reverse Na+/Ca2+ exchange. Glia 62(7):1162–1175
Bechtold DA, Kapoor R, Smith KJ (2004) Axonal protection using flecainide in experimental autoimmune encephalomyelitis. Ann Neurol 55(5):607–616
Morsali D, Bechtold D, Lee W et al (2013) Safinamide and flecainide protect axons and reduce microglial activation in models of multiple sclerosis. Brain 136(Pt 4):1067–1082
Lo AC, Black JA, Waxman SG (2002) Neuroprotection of axons with phenytoin in experimental allergic encephalomyelitis. Neuroreport 13:1909–1912
Bechtold DA, Miller SJ, Dawson AC et al (2006) Axonal protection achieved in a model of multiple sclerosis using lamotrigine. J Neurol 253:1542–1551
Craner MJ, Damarjian TG, Liu S et al (2005) Sodium channels contribute to microglia/macrophage activation and function in EAE and MS. Glia 49(2):220–229
Counihan TJ, Duignan JA, Gormley G et al (2014) Does long-term partial sodium channel blockade alter disease progression in MS? Evidence from a retrospective study. Ir J Med Sci 183(1):117–121
Gnanapavan S, Grant D, Morant S et al (2013) Biomarker report from the phase II lamotrigine trial in secondary progressive MS- neurofilament as a surrogate of disease progression. PLoS One 8(8):e70019
Kapoor R, Furby J, Hayton T et al (2010) Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol 9(7):681–688
Kleinewietfeld M, Manzel A, Titze J et al (2013) Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496(7446):518–522
McGuire S (2010) Institute of Medicine. 2010. Strategies to reduce sodium intake in the United States. The National Academies Press, Washington. Adv Nutr 1:49–50
Appel LJ, Frohlich ED, Hall JE et al (2011) The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the American Heart Association. Circulation 123:1138–1143
Brown IJ, Tzoulaki I, Candeias V et al (2009) Salt intakes around the world: implications for public health. Int J Epidemiol 38:791–813
Junger WG, Liu FC, Loomis WH et al (1994) Hypertonic saline enhances cellular immune function. Circ Shock 42:190–196
Machnik A, Neuhofer W, Jantsch et al (2009) Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15(5):545–552
Korn T, Bettelli E, Oukka M et al (2009) IL-17 and Th17 Cells. Annu Rev Immunol 27:485–517
Ghoreschi K, Laurence A, Yang XP et al (2010) Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 467:967–971
Wu C, Yosef N, Thalhamer T et al (2013) Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496(7446):513–517
Codarri L, Gyülvészi G, Tosevski V et al (2011) RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 12:560–567
El-Behi M, Ciric B, Dai H et al (2011) The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol 12:568–575
Reboldi A, Coisne C, Baumjohann D et al (2009) C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 10:514–523
Go WY, Liu X, Roti MA et al (2004) NFAT5/TonEBP mutant mice define osmotic stress as acritical feature of the lymphoid microenvironment. Proc Natl Acad Sci USA 101:10673–10678
Farez MF, Fiol MP, Gaitán MI et al (2015) Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 86(1):26–31
Fulgoni VL 3rd (2007) Limitations of data on fluid intake. J Am Coll Nutr 26(5 Suppl):588S–591S
Han MH, Hwang SI, Roy DB et al (2008) Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 451(7182):1076–1081
Goodfriend TL, Elliott ME, Catt KJ (1996) Angiotensin receptors and their antagonists. N Engl J Med 334:1649–1654
Stegbauer J, Lee DH, Seubert S et al (2009) Role of the renin-angiotensin system in autoimmune inflammation of the central nervous system. Proc Natl Acad Sci USA 106:14942–14947
Maillard P, Seshadri S, Beiser A et al (2012) Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol 11:1039–1047
Platten M, Youssef S, Hur EM et al (2009) Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc Natl Acad Sci USA 106(35):14948–14953
Hucke S, Eschborn M, Liebmann M et al (2016) Sodium chloride promotes pro-inflammatory macrophage polarization thereby aggravating CNS autoimmunity. J Autoimmun 67:90–101
Yi B, Titze J, Rykova M et al (2015) Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res 166(1):103–110
Jörg S, Kissel J, Manzel A et al (2016) High salt drives Th17 responses in experimental autoimmune encephalomyelitis without impacting myeloid dendritic cells. Exp Neurol 279:212–222
Krementsov DN, Case LK, Hickey WF et al (2015) Exacerbation of autoimmune neuroinflammation by dietary sodium is genetically controlled and sex specific. FASEB J 29(8):3446–3457
Zhou X, Zhang L, Ji WJ et al (2013) Variation in dietary salt intake induces coordinated dynamics of monocyte subsets and monocyte-platelet aggregates in humans: implications in end organ inflammation. In: Zirlik A (ed) PLoS One 8(4):e60332
Hernandez AL, Kitz A, Wu C et al (2015) Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Investig 125(11):4212–4222
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of this paper.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zostawa, J., Adamczyk, J., Sowa, P. et al. The influence of sodium on pathophysiology of multiple sclerosis. Neurol Sci 38, 389–398 (2017). https://doi.org/10.1007/s10072-016-2802-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-016-2802-8