Abstract
Vitamin D insufficiency or deficiency (VDD) is a very prevalent condition in the general population. Vitamin D is necessary for optimal bone mineralization, but apart from the bone effects, preclinical and observational studies have suggested that vitamin D may have pleiotropic actions, whereas VDD has been linked to several diseases and higher all-cause mortality. Thus, supplementing vitamin D has been considered a safe and inexpensive approach to generate better health outcomes—and especially so in frail populations. Whereas it is generally accepted that prescribing of vitamin D in VDD subjects has demonstrable health benefits, most randomized clinical trials, although with design constraints, assessing the effects of vitamin D supplementation on a variety of diseases have failed to demonstrate any positive effects of vitamin D supplementation. In this narrative review, we first describe mechanisms through which vitamin D may exert an important role in the pathophysiology of the discussed disorder, and then provide studies that have addressed the impact of VDD and of vitamin D supplementation on each disorder, focusing especially on randomized clinical trials and meta-analyses. Despite there already being vast literature on the pleiotropic actions of vitamin D, future research approaches that consider and circumvent the inherent difficulties in studying the effects of vitamin D supplementation on health outcomes are needed to assess the potential beneficial effects of vitamin D. The evaluation of the whole vitamin D endocrine system, rather than only of 25-hydroxyvitamin D levels before and after treatment, use of adequate and physiologic vitamin D dosing, grouping based on the achieved vitamin D levels rather than the amount of vitamin D supplementation subjects may receive, and sufficiently long follow-up are some of the aspects that need to be carefully considered in future studies.
Similar content being viewed by others
1 Introduction
Vitamin D insufficiency or deficiency (VDD) have been identified as very prevalent conditions in the general population, with some authors coining the use of the terms of “vitamin D deficiency epidemic, or pandemic” [1, 2]. Other than the well-known effects of vitamin D on bone metabolism, vitamin D exerts pleiotropic actions. On one hand, VDD is associated with a series of adverse health conditions; on the other, supplementation with vitamin D is a low‐cost and safe intervention, making it an attractive therapeutic option in the clinician’s and researcher’s armature. These facts have contributed to the “explosion” in the interest of the scientific community on the understanding of the pleiotropic actions of vitamin D, among which is its immunomodulating effects.
Currently, there is vast research on the effects of vitamin D on human homeostasis, mechanisms of action, and supplementation outcomes. Up to June 2022, a PUBMED (MeSH) search on vitamin D yielded 65,758 results, with abrupt increases in the scientific publications in the last two decades. However, many of the published studies that have linked decreased vitamin D levels with poorer health outcomes are of associative nature, making the evidence whether vitamin D per se contributes or not to poor health relatively weak. In this narrative review, we present the links between VDD and a variety of diseases such as infections, COVID-19, type 2 diabetes (T2D), hypertension, cardiovascular, gastrointestinal, neurodegenerative and autoimmune diseases, and also the impact of vitamin D supplementation. First, we describe briefly, the mechanisms through which vitamin D could have an impact on the discussed pathology (Fig. 1). Then, we provide the available evidence from purely an association point of view. Since association does not prove causation, and there are often undetected confounders in reported associations, we then focused on meta-analyses and systematic reviews where vitamin D administration has been tested in the treatment or prognosis of the disease in question. Original articles and/or meta-analyses on the supplementation of vitamin D outcomes are also provided if available.
1.1 Regulation of Vitamin D
Vitamin D exists in two major forms: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol); the former is obtained with diet mainly from fungi and also plants, whereas the latter can be either obtained with diet (animal products) or synthesized in the skin from the conversion of the cholesterol precursor 7-dehydrocholesterol after exposure to adequate ultraviolet B radiation. Sun exposure for vitamin D synthesis may be efficient only when the angle of sun rays is more than 45°. As a result of this, inhabitants of the northern hemisphere do not receive sufficient amounts of vitamin D through skin synthesis during winter months, and in some northern areas, defective sun exposure may last up to 6 months of the year [3]. Moreover, a typical Western diet is poor in vitamin D [4]. To increase vitamin D ingestion, some countries have applied a policy of enriching milk products [5, 6] and margarine [7] with vitamin D, while also the use of light bulbs for artificial UVB exposure is another tool to increase vitamin D synthesis.
Vitamin D needs to undergo activation, which consists of two consecutive hydroxylations; the first in the liver and the second predominantly in the kidneys, but also in extrarenal tissues. In the liver, cholecalciferol is quickly hydroxylated by the enzyme 25-hydroxylase (a CYP450-dependent enzyme also known as CYP2R1) yielding 25-hydroxyvitamin D (25(OH)D) in an uncontrolled process [8]. Low plasma calcium or phosphate levels regulate parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF-23) levels, leading to the 1α-hydroxylation of 25(OH)D in the kidney and particularly in the mitochondria of the proximal convoluted tubule cells by the 1-hydroxylase enzyme (CYP27B1), resulting in the active vitamin D (1,25(OH)2D) [9] (Fig. 2). The 1α-hydroxylation may also occur in extrarenal tissues (epithelial tissues, placenta, bone, endocrine glands, brain, liver, and endothelium [10, 11]), and especially in immune cells [12]. The 1,25(OH)2D can then de-activate 1α-hydroxylase and stimulate the 24-hydroxylase enzyme, which destroys 25(OH)D, providing a negative feedback loop that controls active vitamin D levels. The 24-hydroxylation of 25(OH)D yields 24,25(OH)2D, the inactive metabolite, the formation of which, along with saturation of the synthesis of vitamin D in the skin, guards against vitamin D intoxication. Even though the active form of vitamin D is 1,25(OH)2D, conventional blood tests measure 25(OH)D because of its long half-life (~15 days) [13], making it a suitable marker of vitamin D storage. In contrast, circulating 1,25(OH)2D does not reflect vitamin D status because of its short half-life of a few hours and its tight regulation by PTH, calcium, and phosphate levels [14]. The direct measurement of free (non-protein bound) 25(OH)D is also possible, with some authors proposing that the contemporaneous assessment of total and free 25(OH)D levels, as well as vitamin D binding protein (VDBP) and PTH should be measured in assessing vitamin D status and the effect of vitamin D supplementation on clinical outcomes [15,16,17,18].
Schematic representation of 1α-hydroxylation of 25 (OH)D in the active form in renal and extrarenal tissues. Several tissues have been described to have the CYP27B1 enzyme responsible for the 1α-hydroxylation of 25 (OH) D, but here emphasis is given in the immune and epithelial cells. Of note is that the control of the CYP27B1 activity differs between renal and extrarenal tissues. FGF23 fibroblast growth factor, IFN-γ interferon gamma, PTH parathyroid hormone, TLR toll-like receptor, TNF-α tumor necrosis factor alpha
The 1,25(OH)2D binds to the vitamin D receptor (VDR), a member of the nuclear receptor family of ligand-regulated transcription factors, which then forms a heterodimer with the retinoid X receptor. The heterodimer enters the cell nucleus and binds to vitamin D responsive elements (VDRE) in DNA, resulting in regulation of the expression of key genes in target organs to yield its actions. This is the basis for the genomic actions of vitamin D. Genomic actions of vitamin D require hours before any effects can be noticed. However, vitamin D also exerts actions that are rapid (within seconds to minutes); these are the nongenomic actions of vitamin D that are yielded without gene activation. The nongenomic actions of vitamin D may occur when vitamin D activates the VDR found outside the nucleus [19]. Furthermore, it has been suggested that vitamin D may also have a membrane receptor, which could explain the rapid nongenomic actions of vitamin D. However, the membrane target of vitamin D is currently not fully elucidated [20].
1.2 The Difficulty in Assessing the Effects of Vitamin D Supplementation in Health Outcomes
Vitamin D is a nutrient, but the major determinant of vitamin D levels is dependent on skin synthesis following sunlight exposure. Thus, placebo-controlled randomized controlled trials (RCTs) assessing the effects of vitamin D on health outcomes differ greatly from standard RCTs using drugs, since it is impossible to exclude vitamin D intake or sunlight exposure in the placebo arms of the vitamin D trials. [21]. Moreover, since VDD is a very prevalent condition, some RCTs (for instance, the large VITAL study [22, 23]) also allow supplementation with low doses of vitamin D in the placebo group. While most RCTs are done in the general population to increase generalizability of the study results, it is well known that anthropometric characteristics of the study participants such as age, body mass index (BMI), and even skin pigmentation may affect the intake or metabolism of vitamin D, and therefore constitute confounders [24, 25]. However, RCTs on vitamin D typically use standard doses of supplementation rather than personalized doses based on the characteristics of the participants. Moreover, in several RCTs, baseline and on-treatment 25(OH)D are not monitored; this is again a great confounder of the study results since subjects on the placebo arm may actually achieve higher 25(OH)D levels compared with subjects on treatment. Even when plasma 25(OH)D levels are monitored, there is large variance in the results, especially if the widely used immunoassay methodology is used [26]. Thus, data from different studies are not always comparable and could not be used in meta-analyses. Finally, the dose-response between vitamin D and its health effects is “S shaped” [21, 27]. This implies that, on one hand, in subjects with VDD, large doses of vitamin D supplementation would be needed to elicit any meaningful effect, while on the other, supplementation in vitamin D replete subjects would not yield any effect. These are important confounders that make the interpretation and the execution of an RCT on vitamin D much more demanding compared with a drug RCT, and are expected to have affected the results of the RCTs that are presented in the following chapters.
2 Classical Vitamin D Actions
2.1 Vitamin D and Bone
Mechanisms Vitamin D exerts both direct and indirect actions on bone [28]. Vitamin D is a major determinant of mineral homeostasis, promoting intestinal calcium and phosphorus absorption, which are required for optimal mineralization of bone. Vitamin D also exerts direct actions on bone. The direct actions of vitamin D on bone are more complex to demonstrate, and studies on VDR or CYP27B1 knockout animal models treated with a rescue high-calcium, high-phosphorus, and high-lactose diet have shown that even though severe bone abnormalities such as rickets (i.e., defective mineralization of the growth plate and adjacent metaphysis in the growing skeleton) and osteomalacia (i.e., the accumulation of unmineralized osteoid at sites other than the growing metaphysis) are prevented [29, 30], changes in osteoblast number, mineral apposition rate, and bone volume remain [31]. Indeed, as reviewed in [28], direct effects of vitamin D on osteoblasts proliferation and survival and in the mineralization process have been shown.
Even though it is well established that acquired or genetic alterations in the vitamin D endocrine system can lead to rickets and osteomalacia and that, vice versa, treatment with an adequate quantity of vitamin D prevents rickets, osteomalacia [32], and renal osteodystrophy, the role of vitamin D in the skeleton of adults and older adults is often disputed.
In the large Vitamin D Assessment (VIDA) study—a trial in which participants were randomized to receive either 100,000 IU vitamin D3 or placebo monthly—correction of severe vitamin D deficiency led to improvement in bone mass density (BMD) [33], whereas vitamin D supplementation in already vitamin D replete adults was not associated with improved bone mass density (BMD) or bone quality [33]. Moreover, no effect was found in the VIDA trial in risk of fractures or falls after vitamin D supplementation in either the whole dataset or the vitamin D deplete group compared with placebo [33]. In the other large RCT Vitamin D and OmegA-3 TriaL (VITAL), supplemental vitamin D3 (2000 IU/d) was compared with placebo. Also in this study, vitamin D supplementation did not affect BMD of the spine, hip, or whole body, and this lack of effect was independent of baseline 25(OH)D levels [23]. However, among subjects with baseline free vitamin D levels below the median (< 14.2 pmol/L), those receiving vitamin D supplementation showed a slight increase in spine aBMD (0.75% versus 0%; p = 0.043) and attenuation in loss of total hip aBMD (−0.42% versus −0.98%; p = 0.044) compared with placebo [23]. In the Calgary study, the long-term outcomes of vitamin D supplementation at 400, 4000, and 10,000 IU per day were compared. It was found that subjects receiving the very high dose of vitamin D supplementation had decreased BMD at the radius and tibia compared with subjects receiving 400 IU daily [34], while no differences in BMD were noted between the 4000 and 400 IU groups. Moreover, very high-dose vitamin D supplementation (4000 and 10,000 IU/day) may result in hypercalciuria and/or hypercalcaemia [34]. The decrease in BMD with very high doses of vitamin D may be due to excessive bone resorption by increasing the number and activity of osteoclasts directly [35], or indirectly through activation of osteoblasts, which in turn activate osteoclastogenesis [36]. Another important aspect related to bone health often evaluated in clinical studies is the risk of fractures. In a large meta-analyses conducted by Bolland et al., administration of vitamin D had no effect on total fracture [36 trials; n = 44.790, relative risk (RR) 1.00, 95% confidence intervals (CI) 0.93–1.07], hip fracture (20 trials; n = 36.655, RR 1.11; 95% CI 0.97–1.26), or falls (37 trials; n = 34.144, RR 0.97; 95% CI 0.93–1.02), and similar results were found when comparing randomized controlled trials (RCTs) of high-dose versus low-dose vitamin D [37]. Moreover, regarding hip fractures, this meta-analysis showed that, whereas there is reliable evidence that vitamin D supplementation does not reduce hip fractures, it is uncertain whether it might increase the risk of hip fractures [37]. On the contrary, a meta-analysis of eight studies including 30,970 participants showed that the combined administration of vitamin D and calcium can reduce the risk of total fractures by 15% [odds ratio (OR) 0.85; 95% CI 0.73–0.98] and the risk of hip fractures by 30% (OR 0.70; 95% CI 0.56–0.87) [38].
2.2 Vitamin D, Muscle Strength, Muscle Mass, Muscle Power, and Risk of Falls
Mechanisms VDD has been associated with musculoskeletal dysfunction, a reduction in muscle strength and size, and increased intramuscular noncontractile tissue [39, 40].
One of the largest meta-analyses evaluated the effect of vitamin D supplementation on muscle strength, including data of 29 RCTs involving 5533 subjects. It demonstrated that vitamin D supplementation had a small but significant effect on improving global muscle strength (SMD 0.17, 95% CI 0.03–0.31, p = 0.02), and in particular there was a significant positive effect of vitamin D supplementation on lower limb muscle strength (SMD 0.19; 95% CI 0.05–0.34; p = 0.01), but not on grip strength (SMD 0.01; 95% CI 0.06–0.07; p = 0.87) [41]. In a subgroup analyses, it was further demonstrated that the improvement in muscle strength was greater in patients who at baseline had 25(OH)D values < 30 nmol/L, compared with those who had 25(OH) D ≥ 30 nmol/L. Moreover, a meta-regression showed a significant association between changes in 25(OH)D concentration and changes in muscle strength [slope 95% CI 0.01 (0.00, 0.01); p = 0.01]. With regards to age, vitamin D supplementation in subjects older than 65 years resulted in a significant improvement of muscle strength (SMD 0.25; 95% CI 0.01–0.48), whereas supplementation in younger people did not (SMD 0.03; 95% CI 0.08–0.14) [41]. This meta-analysis also assessed the effects of vitamin D supplementation on muscle mass and muscle power, even though a limited number of studies had assessed these outcomes (six and five studies, with a total of only 538 and 245 subjects, respectively). It was shown that vitamin D supplementation does not improve muscle mass or muscle power [41].
An improvement in lower limb muscle strength could be a promising mechanism through which vitamin D supplementation could reduce the risk of falls, since, on one hand, quadriceps strength is a significant predictor of falls [42] and, on the other hand, VDD has also been linked to an increased risk of falls [43, 44]. Thus, whether vitamin D supplementation confers protection from falls has received a lot of interest, but meta-analyses on this topic have yielded conflicting results. Early meta-analyses reported beneficial effects of vitamin D supplementation on reducing falls, and two analyses reported that vitamin D supplementation combined with calcium, but not vitamin D supplementation alone, reduces the risk of falls [43, 45]. However, subsequent meta-analyses reported neutral effects of vitamin D supplementation on falls [46], and when very high doses of vitamin D supplementation were used, there was an increased risk of falls [47, 48].
In a 2014 trial, a sequential meta-analysis approach to reduce the risk of false positive effects, Bolland et al. analyzed data from 20 RCTs (n = 29,535). They reported that vitamin D supplementation did not reduce the relative risk for falls by 15% or more, and similar null effects were reported when they performed a sensitivity analysis, reducing the risk reduction threshold at 10% [49]. There were no differences in the effects of vitamin D supplementation alone or vitamin D and calcium supplementation on the risk of falls. Based on their approach, the authors concluded that it is unlikely that similar future trials may alter these negative conclusions of vitamin D supplementation on the rick of falls [50]. The null effects of vitamin D supplementation on reducing the risk of falls were replicated in a subsequent meta-analysis of the same group in 2018, including data of 37 trials and a total of 34,144 subjects (RR 0.97; 95% CI 0.93–1.02). Of note is that in this meta-analysis, vitamin D supplementation did not decrease the RR of falls by 7.5%—i.e., the efficacy of vitamin D supplementation at a lower RR threshold was tested but still no clinically meaningful effect of vitamin D supplementation on reducing the risk of falls was found [37].
3 Non-Classical Vitamin D Actions
3.1 Vitamin D and Hypertension
Mechanisms Preclinical studies have shown that VDD may predispose to hypertension through upregulation of the renin–angiotensin–aldosterone system (RAAS) and increased vascular resistance and vasoconstriction [51,52,53]. On the other hand, VDR activation has been shown to inhibit intrarenal mRNA levels and protein expression of key components of the RAAS [51].
Evidence shows that vitamin D supplementation is effective in reducing blood pressure in patients with hypertension and VDD [54]. Once again, the modality of vitamin D supplementation impacts the outcome, with daily [55,56,57] or weekly [58] administrations of vitamin D improving hypertension outcomes, whereas large bolus vitamin D dosing (e.g., 100,000 IU VD every 2 months) failed to reduce blood pressure in vitamin D deficient subjects [59]. Large doses of vitamin D might also have detrimental vascular effects, since they can result in vascular calcification [60]. On the contrary, vitamin D supplementation in vitamin D replete subjects has null effects on lowering blood pressure [61]. Antihypertensive medications may also affect whether vitamin D supplementation will affect blood pressure. For instance, Bernini et al. did not find any effect of acute or chronic vitamin D supplementation on RAAS in patients with essential hypertension on RAAS inhibitor treatment [55]; however, they also showed that chronic vitamin D receptor activation in drug-free essential hypertensives suppresses RAAS components [62]. This evidence further underlines that the blood pressure effects of vitamin D in humans are dependent on the activity of RAAS.
Low serum 25(OH)D levels have also been associated with an increased risk of developing hypertension [53], which raises the question of whether vitamin D supplementation can impact the incidence of hypertension, and this is of great clinical interest. It is important to note that to evaluate the effects of vitamin D supplementation on the incidence of chronic diseases, such as hypertension, the intervention period should be long enough (> 5 years) to record a sufficient number of events [54].
However, in the VITAL study (intervention for 5 years), vitamin D supplementation did not reduce the incidence of cardiovascular events [63], and there was no specific mention of whether the incidence of hypertension was affected. DO-HEALTH was a RCT on adults aged 70 years or older without major comorbidities. Treatment with 2000 IU/day of vitamin D did not improve systolic (SBP) or diastolic blood pressure (DBP) compared with placebo [64]. However, as the authors pointed out, in this trial only 40.7% of individuals had 25(OH)D levels less than 20 ng/ml at baseline, and all participants were allowed to take up to 800 IU/day of vitamin D outside the study medication [64].
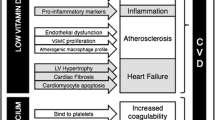
3.2 Cardiovascular Events
Mechanisms VDR is expressed in endothelial cells, vascular smooth muscle cells, and cardiac myocytes [65]. Vitamin D preserves endothelial function through inhibition of the proliferation of vascular smooth muscle cells [66], and also reduces oxidative stress, inflammation, and thrombogenesis [67]. It has also been suggested that it can modify lipid metabolism by increasing the activity of lipoprotein lipase in adipose tissue [68] and by reducing fatty acid absorption [69]. As discussed earlier, it can also reduce RAAS activity, thereby decreasing blood pressure.
In a meta-analysis of nearly 850,000 individuals, patients were divided into tertiles for 25(OH)D supplementation. Patients on the lower tertile of 25(OH)D concentrations had an increased risk of death from cardiovascular disease compared with patients on the top thirds of 25(OH) D concentrations (RR 1.35; 95% CI 1.13–1.61) [70]. Moreover, another meta-analysis showed that subjects in the lowest quintile of 25(OH)D concentration had an increased risk of cardiovascular mortality compared with subjects in the highest quintile (RR 1.41, 95% CI 1.18–1.68 in subjects without a history of cardiovascular disease and RR 1.65, 95% CI 1.22–2.22 in subjects with a history of cardiovascular disease) [71]. In a recent large cohort study in 24,311 patients with T2D and 67.789 subjects with prediabetes (i.e., a study population with high CVD risk) it was shown that 25(OH)D levels were inversely and independently associated with the risk of incident cardiovascular outcomes and all-cause mortality. Moreover, in a recent large cohort study in 24,311 patients with T2D and 67,789 subjects with prediabetes (i.e.. a study population at increased risk for CVD [72]), 25(OH)D was associated with lower risk of incident CVD events and mortality [73]. In a dose-response analysis, it was shown that increasing 25(OH)D up to 50–60 nmol/L decreased mortality and cardiovascular events [73].
However, in the two large RCTs (VITAL and VIDA) with long follow-up, supplementation with vitamin D did not impact on major cardiovascular events or cardiovascular death compared with placebo [63, 74]. The same conclusion was reached by Barbarawi and colleagues analyzing data of 21 RCTs with a total of 83,000 individuals [75].
Whether vitamin D supplementation affects the risk factors for CVD has also been investigated. Earlier systematic reviews and meta-analyses have reported a null effect of vitamin D supplementation on the modification of CVD risk factors [49, 76,77,78]. Mirhosseini et al. recently performed a systematic review and meta-analysis with stringent inclusion criteria, including only studies in which the duration of vitamin D supplementation was at least 3 months; studies using a daily, weekly, or monthly frequency of vitamin D dosage; and studies where baseline and post-intervention serum 25 (OH)D levels were included. Eighty-one studies met the selection criteria. The authors showed that supplementation of vitamin D led to a reduction of SBP and DBP, a reduction of total cholesterol and triglycerides, an increase in HDL, and a reduction in high-sensitivity C-reactive protein (hs-CRP) [79]. In subgroup analyses, they also reported dose-effect responses comparing studies in which subjects received ≥ 4000 IU/day with studies in which patients received < 4000 IU/day. They showed that trials with vitamin D supplementation ≥ 4000 IU/day had greater reductions in SBP, DBP, and hs-CRP. Similar effects were reported when serum 25(OH)D levels higher or lower than 86 nmoL/L were considered, with subjects with higher 25(OH)D levels showing greater reductions in SBP, DBP, and hs-CRP. On the contrary, lipid changes were not associated with the dose or the achieved serum 25(OH)D concentrations [79]. As the authors state, the discrepancy with earlier systematic reviews and meta-analyses could be attributed to the quality of the studies included (small sample sizes, too low doses of vitamin D supplementation, and too narrow intervention length). The effect of vitamin D supplementation on markers of arterial stiffness [i.e., pulse wave velocity (PWV) and augmentation index (AI)] was also assessed, but the numbers of studies that evaluated these markers was small (11 and 10 studies, respectively). While there were no overall effects of vitamin D supplementation on these markers, subgroup analyses found that AI was lower in patients with serum 25(OH)D concentrations ≥ 86 nmol/L and in patients receiving vitamin D doses ≥ 4000 IU/day, with the authors concluding that vitamin D supplementation may improve the markers of arterial stiffness [79]. These results seem in line with the results of a prespecified analysis of a subsample of participants in the Vitamin D Assessment (VIDA) study who underwent suprasystolic oscillometry [74]. The VIDA study showed that monthly high-dose (i.e., 100,000 IU/month) supplementation with vitamin D led to improvements in AI, PWV, peak reservoir pressure, and backward pressure amplitude [74]. Aortic systolic blood pressure also improved, whereas SBP and DBP showed only small, nonsignificant reductions [74].
3.3 Acute Respiratory Tract Infection and Influenza
Mechanisms Vitamin D is involved in the control of both the innate and adaptive immune response. Virtually all immune cells express VDR and CYP27B1, and it has been shown that macrophages, activated T and B cells, dendritic cells, and endothelial cells lining the upper and lower respiratory tracts can hydroxylate 25(OH)D into the active form[80,81,82]. Neutrophils express VDR, but it seems that they do not possess CYP27B1 [83]. Evidence suggests that 1,25(OH)2D controls the innate immune response through a negative feedback loop on macrophages and other immune cells. More specifically, IFNγ-activated macrophages induce 1,25(OH)2D release, which in turn activates VDR on macrophages, suppressing the expression of key genes producing proinflammatory proteins [84]. Regarding regulation of adaptive immune responses, 1,25(OH)2D has been shown both to inhibit proliferation and differentiation of activated human B cells [85], to inhibit T helper cells, and also to promote Treg cells [86]; the net outcome of these effects would be to limit inflammatory processes. In the specific case of influenza virus, it has been shown that incubation of human lung A549 epithelial cells with 1,25(OH)2D before or after exposure to influenza A virus led to decreased production of TNF-α, IFN-β, and IFN-stimulated gene-15, and downregulated interleukin (IL-8 and IL-6 RNA levels [87]. An extensive review of the mechanisms through which vitamin D modulates and controls the immune responses has been performed recently [81].
A negative linear association among vitamin D levels and lung infections and function has been established in a large cross-sectional study of 6789 subjects, where for each 10 nM/L increase in vitamin D levels, the risk of infection was reduced by 7% [88]. Negative associations between vitamin D levels and the risk [89] or severity of pneumonia have also been described [90].
Urashima et al. performed an RCT in children (N = 167 on vitamin D and N = 167 on placebo) receiving either a daily supplement of vitamin D (1200 IU/day) or placebo. They found that patients treated with vitamin D had a lower incidence of influenza A compared with placebo (incidence of influenza A, 10.8% in the vitamin D group versus 18.6% in the placebo group; RR 0.58; 95% CI 0.34–0.99; p = 0.04) [91]. Apart from these positive outcomes of higher vitamin D levels and of vitamin D supplementation on influenza and other lung infections, other studies have reported neutral [92] or even negative results [93] of vitamin D supplementation on the outcomes of lung infections. It is not clear if this discrepancy is due to methodological issues [low vitamin D supplementation [92] or weak endpoints used (questionnaires on self-reported symptoms) [93]], characteristics of the study population, or are dependent on the baseline vitamin D status. For instance, in another RCT conducted by Urashima et al. investigating the effects of vitamin D supplementation during the 2009 H1N1 pandemic, they showed that subjects in the vitamin D group (2000 IU/day) had a lower incidence of influenza A or B compared with the placebo group during the first month of intervention, whereas there was a higher incidence of infection during the second month [94]. It would be tempting to hypothesize that at the beginning of the intervention, vitamin D levels were low, allowing the treatment to show a positive protective effect, whereas once vitamin D levels were restored, the vitamin D had no impact in the prevention of infection. Unfortunately, in this study, serum levels of 25(OH)D were not measured, which could have explained the reasons for this difference at the two time periods, and thus this suggestion is speculative. A meta-analysis of 25 RCTs (including a total of 10,933 participants) supports the protective effects of vitamin D on acute lung infections. More specifically, vitamin D supplementation reduced the risk of acute respiratory infections among all participants [adjusted OR (aOR) 0.88; 95% CI 0.81–0.96; heterogeneity p < 0.001]. Importantly, the protective effects were seen in individuals receiving daily or weekly vitamin D (aOR 0.81;95% CI 0.72–0.91), but not in those receiving bolus doses (aOR 0.97; 95% CI 0.86–1.10; p = 0.05). Moreover, among subjects receiving daily or weekly vitamin D, protective effects of vitamin D were stronger in those who baseline 25(OH)D concentrations < 25 nmol/l (aOR 0.30; 95% CI 0.17–0.53) compared with those with baseline 25(OH)D ≥ 25 nmol/L (aOR 0.75; 95% CI 0.60–0.95; p for interaction = 0.006) [95]. In a more recent meta-analysis by the same group, including data from 43 RCTs and a total of 48,488 participants, the protective effect of vitamin D supplementation when given using a daily dosing regimen, at daily dose equivalents of 400–1000 IU on acute respiratory infections was confirmed [96].
3.4 Tuberculosis
Vitamin D was used in the pre-antibiotic era for the treatment of patients with tuberculosis (TB), when the ancient Greeks had first introduced “heliotherapy” (i.e., sunlight exposure) to treat TB [97]. Moreover, in preclinical studies, it has been shown that 1,25(OH)2D induces antimycobacterial activity in vitro in monocytes and macrophages [98, 99]. However, recent controlled trials and meta-analyses have produced either minimal or null effects in a variety of TB-associated outcomes. A systematic review showed that serum vitamin D levels are not associated with the incidence of latent tuberculosis infection [100]. As the authors pointed out, different 25(OH)D assays were used in the studies included, which have differences in their sensitivity and precision, and that may have affected the results of the meta-analysis. In a RCT on TB contacts, it was shown that a single dose of 2.5 mg vitamin D (i.e., 100,000 IU) suppressed recombinant Mycobacterium growth through Bacillus Calmette–Guérin (BCG)-lux analysis at 24 h but not at 96 h, suggesting improved innate but unmodified acquired immunity against mycobacteria compared with placebo [101]. In a large RCT on children with a negative Quantiferon test at randomization, supplementation with a weekly dose of 14,000 IU vitamin D for 3 years did not result in a lower risk of tuberculosis infection, tuberculosis disease, or acute respiratory infection compared with placebo [102]. Finally, in the, thus far, largest meta-analysis investigating the effects of vitamin D supplementation on patients with pulmonary TB, vitamin D supplementation resulted in an increase in lymphocyte count, an improvement in chest radiography (mean number of zones involved), and an increased proportion of sputum smear and culture conversion. On the contrary, compared with placebo, vitamin D yielded null effects on time to sputum smear and culture conversion, and on mortality [103].
3.5 COVID-19
Considering the previous implications of vitamin D in acute respiratory tract infections, soon after the outbreak of the COVID-19 pandemic the research community started investigating whether vitamin D supplementation may have an impact in preventing infection with Severe acute respiratory syndrome coronavirus (SARS-COV2), or on the severity of COVID-19. This was especially important at the beginning of the pandemic when the medical community had almost no treatments in the fight against COVID-19.
Mechanisms Several mechanisms have been proposed through which vitamin D could offer protection against COVID-19. First, by regulating the innate immune response, vitamin D induces the production of the antimicrobial peptides cathelicidin (or LL-37) and β defensin, blocking the viral entry into cells [104]. Because of the actions of vitamin D on the adaptive immune system, and specifically the shift away from a proinflammatory state, it reduces the risk of cytokine storm, which is particularly detrimental in severe cases of COVID-19 [105]. Finally, through regulation of the renin–angiotensin–aldosterone system (RAAS), it suppresses the angiotensin converting enzyme (ACE) while it induces ACE2, leading to a reduction of angiotensin 2 and an increase in angiotensin 1–7. These enzymatic changes restore the ACE: ACE2 imbalance induced by SARS-CoV-2 infection and reduce the risk of vasoconstriction and acute respiratory distress syndrome (ARDS) [105].
Observational studies have shown that patients with VDD have an increased risk for COVID-19 [106], and in the, thus far, largest observational study, we have shown that vitamin D insufficiency or deficiency is associated with a 2.3–3.6 times higher risk of severe COVID-19, necessitating hospital admission [107].
A small, nonrandomized study showed that administration of high doses of vitamin D before SARS-CoV-2 infection was associated with less severe COVID-19 and better survival in older frail patients [108]. Castillo and colleagues performed a pilot study on 76 consecutive patients hospitalized for COVID-19 [109]. Patients at admission and on top of optimal medical treatment were randomized in a 2:1 ratio to receive or not high doses of calcifediol. It was shown that calcifediol supplementation significantly reduced the need for intensive care unit (ICU) treatment [109]. On the contrary, Murai and colleagues randomized 240 subjects to receive either a 200,000 IU vitamin D bolus or placebo. Mean time lag from symptom onset to randomization was relatively long (i.e., mean of 10.3 days). They found that there was no difference in in-hospital stay length, mortality, admission to ICU, or need for mechanical ventilation between the vitamin D and placebo groups [110]. These (negative) results were also confirmed in a post hoc analysis involving only patients with VDD at baseline (N = 115) [110]. In a systematic meta-analysis of our group, including data from nine studies and a total of 2078 patients, we found that vitamin D supplementation was associated with a significant reduction in the need for ICU admission, whereas vitamin D supplementation did not confer protection from COVID-19 mortality [111]. These results are essentially in line with a previous meta-analysis conducted by Shah et al., which was performed earlier and thus had a smaller sample size (N = 532) of COVID-19 patients [112]. Moreover, in our study we performed a meta-regression analysis to identify the effect of dose supplementation; although no significant relationship was found between the dose of supplementation and either severity of disease or mortality, it was shown that low versus high vitamin D supplementation protected from severe disease requiring admission to ICU [111].
In the systematic review and meta-analysis by Pal et al. [113] including data from 13 studies, it was shown that supplementation with vitamin D was associated with improved clinical outcomes in COVID-19 (including mortality) patients, especially when vitamin D is administered in patients after the diagnosis of COVID-19. Based on this finding, the authors suggested that vitamin D can be used as a potential treatment addition in patients with COVID-19. However, it should be noted that in their analysis, only three studies were included where vitamin D supplementation was given before COVID-19 diagnosis [113]. Overall, the discrepancies in the results of vitamin D supplementation on COVID-19 outcomes may have been affected by relatively small sample sizes, and patient’ heterogeneity.
In a recently published phase 3 RCT (CORONAVIT) the investigators assessed the effect of vitamin D supplementation for 6 months on the incidence of all-cause acute respiratory tract infection and COVID-19 [114]. In this study, a test-and-treat approach was selected in which only subjects with 25(OH)D levels < 75 mmol/L were enrolled to receive low (800 IU/day) or high (3200 IU/day) vitamin D supplementation, and were compared with subjects who were not offered vitamin D supplementation (in the intention to treat, N = 1515, 1515, and 2949 for the low dose, high dose, and no supplementation, respectively). It was found that correction of suboptimal vitamin D levels with either supplementation dose was not associated with a reduction in risk of all-cause acute respiratory tract infection or infection from COVID-19 [114].
3.6 Type 2 Diabetes (T2D)
Mechanisms Preclinical studies have shown that vitamin D may modulate β-cell growth and differentiation, enhance insulin secretion [115, 116], increase the expression of the insulin receptor [117], and enhance insulin-mediated glucose transport [118].
However, studies in humans assessing the effect of vitamin D supplementation on insulin secretion and insulin action with gold standard methods have not confirmed these findings. More specifically, in the Tromsö study, a case-control and RCT study, 104 nondiabetic subjects with low serum 25(OH)D levels at baseline were randomized to receive either 20,000 IU twice weekly or placebo. A hyperglycemic clamp was performed at baseline and 6 months after treatment, showing that vitamin D supplementation did not increase first- or second-phase insulin secretion, or insulin sensitivity (assessed as the insulin sensitivity index, ISI) compared with placebo [119]. Similar null effects of vitamin D on insulin secretion (assessed with the intravenous glucose tolerance test, IVGTT) were reported after 3 months of vitamin D supplementation on nondiabetic subjects with low baseline 25(OH)D receiving 50,000 IU/week compared with placebo [120]. These results were confirmed in a meta-analysis that included 12 RCTs and a total of 1181 participants with BMI > 23 kg/m2. It was shown that vitamin D supplementation did not modify whole-body insulin sensitivity (assessed by the HOMA-IR)[121]. Of note, tissue-specific insulin sensitivity may also be assessed using fluorodeoxyglucose positron emission tomography studies in conjunction with a euglycemic hyperinsulinemic clamp [122,123,124,125], but to the best of our knowledge, thus far, it has not been assessed whether there is any correlation between the vitamin D status and tissue-specific insulin sensitivity, or whether vitamin D supplementation may affect tissue-specific insulin sensitivity.
Several association studies have shown an inverse association among serum 25(OH)D levels and fasting glucose [126, 127], glycated hemoglobin (HbA1c)1c [128], insulin resistance, and prevalence of T2D [129].
In a large RCT in patients with prediabetes at a high risk of progression to T2D, supplementation with 4000 IU/day of vitamin D led to a nonsignificant tendency to slower progression to T2D compared with placebo. However, in a post hoc analysis on patients without obesity, severe vitamin D deficiency at baseline and excellent adherence to treatment during the intervention period, a significant effect in decreasing the progression to T2D was seen [130]. This finding was confirmed in two recent systematic reviews and meta-analyses. In a meta-analysis by Barbarawi et al., data from nine RCTs and a total of 43,559 patients were assessed. While in the whole population vitamin D supplementation did not affect the incidence of T2D, post hoc analyses according to the vitamin D dosage showed that subjects receiving ≥ 1000 IU/day had significantly lower incidence of T2D (RR 0.88; 95% CI, 0.79–0.99; p = 0.03). Moreover, patients without obesity who received high-dose treatment had a lower relative risk of T2D (RR 0.68; 95% CI 0.53–0.89; p = 0.005), while no benefit was seen in patients with obesity [131]. In the study by Zhang et al. analyzing data of eight RCTS and 4896 participants, vitamin D supplementation reduced the incidence of T2D (RR 0.89; 95% CI 0.80–1.00; p = 0.04). Similarly to the results of Barbarawi et al., subgroup analyses showed that vitamin D supplementation lowered the risk of new-onset T2D only among non-obese patients, whereas a difference with respect to dose received was not reported [132]. The authors also reported that from five trials in 1080 participants, reversion from prediabetes to normoglycemia was significantly increased by vitamin D supplementation (RR 1.48; 95% CI 1.14–1.92) [132].
The effect of vitamin D supplementation on glycemic control in patients with T2D has also been assessed. Wu et al. assessed 24 studies; supplementation of vitamin D improved HbA1c levels [standardized mean difference (SMD) −0.25 (−0.45 to −0.05)] and this effect was larger among patients with vitamin D deficiency at baseline [SMD −0.39 (−0.67 to −0.10)] and in patients with BMI < 30 kg/m2 [SMD −0.30 (−0.54 to −0.07)] [133]. On the contrary, a subsequent systematic review and meta-analysis by Li and colleagues showed that vitamin D supplementation did not influence fasting blood glucose, HbA1c, or fasting insulin levels, whereas HOMA-IR (i.e., an index of insulin resistance) was improved [134].
There is also evidence that VDD is associated with gestational diabetes mellitus (GDM). In a meta-analysis including seven observational studies and a total of 2146 subjects, of whom 433 developed GDM, it was shown that 25(OH)D levels < 50 nmol/L were associated with development of GDM (OR 1.61; 95% CI 1.19–2.17; p = 0.002) [135]. In a recent systematic review and meta-analysis in a small number of women, supplementation with 2000 IU of vitamin D per day did not affect the incidence of GDM compared with placebo (N = 95 on vitamin D and N = 88 placebo). However, in seven studies including a total of 1722 women comparing the effect of vitamin D supplementation > 2000 IU/day and ≤ 2000 IU/day, it was shown that the incidence of GDM was reduced in the group receiving > 2000 IU of vitamin D per day (RR = 0.70; 95% CI 0.51–0.95; p = 0.02) [136].
3.7 Diabetic Neuropathy and Diabetic Foot Ulcers (DFU)
Mechanisms The role of vitamin D in the function of peripheral nervous system has not been extensively studied [137]. Studies have suggested that vitamin D may be involved in pain perception [138] and that it can induce nerve-growth factor synthesis in human cell lines [139]. Low vitamin D levels have been also reported to impair the differentiation and proliferation of keratinocytes and skin fibroblasts, and to delay DFU healing [140,141,142]. Vitamin D has been shown to induce production of antimicrobial peptides in keratinocyte cells from DFU [143]. Preclinical studies have shown that topical application of vitamin D promotes wound healing in a dose-dependent manner [144], and activates the expression of angiogenic molecules in keratinocytes and the migration of endothelial and keratinocyte cells in a diabetic foot ulceration model [145].
Studies have shown that VDD is associated with painful diabetic neuropathy, diabetic foot ulceration, and diabetic foot infections [146,147,148]. Two recent meta-analyses including a total of 1115 and 1644 patients with T2D showed that severe VDD [i.e., 25(OH) D < 10 ng/ml] is associated with increased risk of diabetic foot ulceration (OR 3.2; 95% CI 2.4–4.3 [149] and OR 3.6; 95% CI 2.9–4.4; p < 0.0001) [150], respectively. In a small RCT on 60 patients with grade 3 DFU according to the “Wagner–Meggit” criteria, patients were randomized to receive either 50,000 IU of vitamin D every 2 weeks or placebo for 12 weeks. Vitamin D supplementation was shown to reduce the ulcer length, width, depth, and erythema rate [151]. A later RCT compared high-dose vitamin D supplementation with 170 μg/day (i.e., 6800 IU) compared with low dose (20 μg/day, i.e., 800 IU) for 48 weeks of treatment. The intention-to-treat analysis showed that patients receiving high-dose supplementation had a higher rate of ulcer healing (70% versus 35%, p = 0.01, in the high versus low supplementation group) [152].
3.8 Neuroprotection
Mechanisms VDR and 1α-hydroxylase are expressed throughout the brain, and they are particularly highly expressed in the substantia nigra and in the hippocampus [153, 154], two important regions for Parkinson’s disease and cognition, respectively. It has been suggested that vitamin D may confer neuroprotection through several mechanisms, including regulation of neurotrophic factors and of nerve growth, protection against cytotoxicity, and reduced oxidative stress [155,156,157]. Vitamin D has also been implicated in the regulation of acetylcholine and clearing of amyloid beta [158].
Considering the high expression of VDR and 1α-hydroxylase in substantia nigra, the impact of VDD on Parkinson’s disease has been studied, yielding conflicting results. In a large prospective study from Finland (N = 3173), patients in the highest quartile for baseline serum vitamin D levels had a 65% lower risk of developing Parkinson’s disease than those in the lowest quartile, suggesting that lower levels of vitamin D in mid-life may increase the risk of Parkinson’s disease [159]. However, later studies in an even larger study sample in the USA failed to confirm this association [160].
The literature regarding vitamin D levels and Parkinson’s disease severity appears more consistent. Cross-sectional studies have consistently reported an association between vitamin D levels and the motor disability in Parkinson’s disease: the lower the serum vitamin D levels, the worse the motor function [161, 162]. However, it is not clear whether vitamin D may modify the severity of the disease, or whether these associations are due to “inverse causality,” since patients suffering worse motor symptoms are also expected to move less and get lower sun exposure.
A small RCT assessed whether high-dose vitamin D supplementation (10,000 IU/day) for 4 months improved balance in patients with Parkinson’s disease compared with placebo. Even though, in the whole dataset, vitamin D supplementation seemed not to have any impact on balance, as measured by the sensory organization test, a post hoc analysis showed that supplementation with vitamin D in younger patients (52–66 years of age) improved balance compared with older participants [163].
With regard to cognitive function in the general population, whereas numerous studies have shown an association between low vitamin D levels and worse cognition [164, 165], intervention studies have failed to show benefits from vitamin D supplementation [165].
The effects of VDD and vitamin D deficiency on multiple sclerosis (MS) have also been studied and they are presented in the chapter regarding autoimmunity.
3.9 Cancer
Mechanisms Early studies have shown that 1,25(OH)2D analogs have potent antiproliferative and pro-differentiating effects on cancer cells in vitro [166]. Also, vitamin D decreases tumor invasiveness, angiogenesis, and metastatic propensity [167, 168].
Systematic reviews and meta-analyses on the levels of vitamin D and mortality outcomes in cancer patients have shown that higher vitamin D levels are protective in a series of cancers such as breast cancer [169], colorectal cancer [170], prostate cancer [171], and hematological malignancies [172]. However, these promising data, based on observational studies, may be biased by a generally better health status and/or a healthier lifestyle (e.g., exercising with greater sunlight exposure) in the subjects who had higher levels of 25(OH)D.
In the large VITAL RCT (N = 25,871), participants were randomized to receive 2000 IU of vitamin D or placebo daily, and omega-3 fatty acids or placebo in a two-by-two factorial design (for a median follow-up time of 5.3 years). Participants had no history of cancer (except nonmelanoma skin cancer) [63]. Supplementation with vitamin D did not significantly reduce the primary endpoint of total invasive cancer incidence (HR 0.96; 95% CI 0.88–1.06), but there was a trend for reducing total cancer mortality (HR 0.83; 95% CI 0.67–1.02) [63]. The authors then accounted for latency, and after excluding events within the first or second year of supplementation, the vitamin D intervention significantly decreased the risk of mortality (HR 0.79; 95% CI 0.63–0.99 after excluding the first and second year cases, respectively). The effect of vitamin D supplementation on cancer mortality was evident in the cumulative incidence curves at 4 years of supplementation. Interestingly, the authors also assessed whether baseline participants’ characteristics could affect the results of the supplementation, and found a significant interaction with BMI, with lean participants having a significant reduction in cancer risk (HR 0.76; 95% CI 0.63–0.90), whereas overweight and obese individuals did not [63].
Earlier RCTs have generally produced null effects of vitamin D supplementation on cancer-related risk reduction, but these studies were either smaller or had methodological problems (low vitamin D supplementation [173, 174] or intermittent bolus dosing [175, 176]). In a meta-analysis, also including the VITAL trial, the protective effect of vitamin D supplementation on cancer mortality was confirmed (HR 0.87; 95% CI 0.79–0.96), whereas there was no effect on cancer incidence (HR 0.98; 95% CI 0.93–1.03) [177].
3.10 Inflammatory Bowel Disease (IBD)
Mechanisms IL-10 knockout mice is an animal model used for the study of IBD; these animals spontaneously develop enterocolitis within 5–8 weeks of birth due to an uncontrolled immune response to resident intestinal flora [178, 179]. People who have an IL-10 gene polymorphism also have an increased risk of developing colitis [180]. In the animal model, it has been shown that VDD exacerbates the symptoms of IBD and increases morbidity and mortality in the affected mice, whereas supplementation with vitamin D improves symptoms and reduces inflammation and mortality [181]. Patients suffering from IBD are at risk for VDD, since they often undergo small-bowel resection, and are treated with cholestyramine to control postresectional diarrhea caused by malabsorprion of bile acids. Both these factors contribute to bile acids loss, which are essential for vitamin D absorption [182]. It has been hypothesized that vitamin D supplementation may reduce inflammation in patients with IBD through decreasing intestinal permeability and increasing the levels of cathelicidin, a peptide that reduces inflammation and promotes healing [183, 184].
A systematic review and meta-analysis on data from 900 IBD patients showed that VDD is a very prevalent condition in these patients, affecting 38.1% of patients with Crohn’s disease (CD) and 31.6% of patients with ulcerative colitis (UC) [185]. Moreover, in a recent systematic review and meta-analysis by Gubatan and colleagues, it was shown that low vitamin D levels were associated with increased odds of clinically active disease and increased odds of clinical relapse among all IBD patients and separately for both CD and UC [186]. Mucosal inflammation and quality of life were also assessed, and it was shown that among all patients, low 25(OH)D levels were associated with increased odds of mucosal inflammation and lower quality of life among all patients and in patients with CD, but not in patients with UC. As the authors argued for the quality of life in UC patients, results may have been underpowered due to the smaller sample size of patients with UC compared with CD in the included studies. On the contrary, the sample sizes were similar for UC and CD regarding the mucosal inflammation outcome, with the authors suggesting that vitamin D may play a specific role in the pathogenesis of CD, and also that VDD may be more suggestive of mucosal inflammation in CD since small bowel inflammation (thus affecting vitamin D absorption) is characteristic of CD but not of UC [186].
Some small studies have assessed the effect of vitamin D supplementation on clinical relapse based on validated scores, serum CRP levels, and quality of life, yielding conflicting results [183, 187]. In a recent, relatively large RCT assessing the effect of vitamin D supplementation on the outcomes of CD using a more robust endpoint (i.e., endoscopic recurrence), 143 patients with CD who had recently undergone ileocecal or ileocolonic resection with ileocolonic anastomosis were randomized to receive 25,000 IU of vitamin D weekly compared with placebo. Even though serum vitamin D levels were doubled in the vitamin D group, the intervention did not affect endoscopic or clinical recurrence compared with placebo [188].
3.11 Autoimmune Disorders
Mechanisms Activation of the VDR by 1,25(OH)2D has been shown to inhibit the differentiation and proliferation of B and T helper lymphocytes, promoting a shift from an inflammatory to a more tolerant immune status [189]. Also, 1,25(OH)2D inhibits the production of proinflammatory Th1 cytokines while stimulating Th2 and regulatory T-cell activity [190]. Independent of VDR activation, 1,25(OH)2D and other vitamin D hydroxyl-metabolites can bind to RORa and RORg, and result in IL17 inhibition [191, 192]. Both these pathways have been implicated in the protective role of vitamin D from autoimmune disorders. An acquired form of vitamin D resistance has also been hypothesized to play a role in the development of autoimmune disorders [193].
VDD has been described in a series of autoimmune disorders, comprising IBD (discussed in the paragraph above), rheumatoid arthritis, Sjogren’s disease, autoimmune thyroiditis, multiple sclerosis (MS), type 1 diabetes, and psoriasis [194,195,196,197]. In this paragraph, we will focus mainly on MS, since the effects of vitamin D on MS have been thoroughly investigated, and to the recent positive findings of the VITAL trial. Of particular interest is also the fact that the CYP27B1 gene, which codes for 25(OH)D 1α-hydroxylase, lies within a genomic region associated with MS, as shown in genome-wide association studies [198]. Indeed, evidence suggests a casual association between genetically induced VDD and increased risk of MS [199, 200].
Several studies have thus shown that patients with MS have lower levels of 25(OH)D compared with healthy subjects [201], and this finding was confirmed in a 2014 systematic review and meta-analysis, including 11 studies and a total of 1007 patients and 829 healthy subjects [202].
Vitamin D has also been used in the treatment of MS, with investigators applying varying doses of vitamin D supplementation from low to extremely high doses. In particular, the “Coimbra protocol” is a protocol of very high doses of vitamin D supplementation, which was originally applied in patients with autoimmune skin disorders (psoriasis and vitiligo) [203]. This protocol has also been applied in MS, with supplementation of vitamin D as high as 1000 IU/kg of body weight per day [193]. A relatively recent systematic review and meta-analysis on the effects of vitamin D supplementation for the treatment of MS has yielded substantially negative results [204]. More specifically, McLaughlin and colleagues evaluated three outcome measures [annualized relapse rate, expanded disability status scale (EDSS) and new gadolinium-enhancing lesions]. Vitamin D supplementation did not improve any of the tested outcomes [204]. However, as the authors discussed in their article, there could be a potential clinically meaningful treatment effect in favor of vitamin D supplementation in the placebo-controlled studies, suggesting that more well-planned and placebo-controlled studies are needed. Of note, in this meta-analysis, high-dose vitamin D supplementation had a significantly worse outcome in terms of relapse rate compared with low dose [204].
In the VITAL trial (i.e., a randomized, double blind, placebo-controlled study with a two-by-two factorial design), the potential benefits of vitamin D supplementation with 2000 IU of cholecalciferol per day with or without of omega 3 fatty acids (1 g/day) on autoimmunity were assessed in 25,871 participants [22]. The mean age of the participants was 67 years. The impact on autoimmunity was assessed by the total confirmed incidence of autoimmune diseases during the 5 years of observation. In particular, annual questionnaires were filled in, inquiring for new onset of rheumatoid arthritis, polymyalgia reumatica, psoriasis, autoimmune thyroiditis, and IBD. They found that subjects on the vitamin D arm had decreased risk for new onset of autoimmune diseases by 22% compared with the placebo group (adjusted HR 0.78; 95% CI 0.61–0.99; p = 0.05). Moreover, after excluding the first 2 years of follow-up to evaluate the latency of the intervention effect, it was confirmed that vitamin D supplementation reduces the incidence of autoimmune diseases and the effect was even stronger (adjusted HR 0.61; 95% CI 0.43–0.86; p = 0.005). Of interest, in a prespecified subgroup analyses, a significant interaction between BMI and the effect of vitamin D supplementation was found with participants with lower BMI being more protected compared with subjects with obesity in whom vitamin D supplementation did not seem to reduce the incidence of autoimmune diseases (adjusted HR 0.47, 95% CI 0.29–0.77 for BMI 18 kg/m2; adjusted HR 0.69, 95% CI 0.52–0.90 for BMI 25 kg/m2; adjusted HR 0.90, 95% CI 0.69–1.19 for BMI 30 kg/m2). Considering the important positive results of this trial, the follow-up period of this study has been extended and more results on the effects of vitamin D supplementation on the incidence of autoimmune diseases are expected.
4 Mendelian Randomization Studies
Bouillon and colleagues reviewed Mendelian randomization studies on the effects of genetically determined low 25(OH)D levels on a variety of conditions such as T2D, cancer, cardiovascular disease, COVID-19, and asthma, showing null effects [205]. Genetic VDD was shown to associate with increased risk for multiple sclerosis [205]. In a very recent study, the approach of Mendelian randomization was also used to assess the association of genetically determined 25(OH)D with mortality [206]. In this study, genetic data of 307,601 participants from the UK Biobank were analyzed, showing evidence of a causal relationship between genetically predicted 25(OH)D and all-mortality outcomes (all-cause, cancer, CVD, and respiratory). More specifically, an L-shaped relationship was described among all-cause mortality, cancer mortality, and CVD mortality with 25(OH)D levels, with the strongest association at concentrations below 25 nmol/L, while the association plateaued at 50 nmol/L. The association of respiratory mortality and 25(OH)D levels was linear [206].
5 Targets for Vitamin D Supplementation
Even though daily vitamin D requirements may be met through synthesis of vitamin D from 7-dehydrocholesterol in the skin after sunlight exposure, deficiency in vitamin D levels is a very common condition. Serum concentrations of 25(OH)D < 10 ng/ml (i.e., 25 nmol/L) are generally indicative of VDD, but the proposed target cut-offs of ideal vitamin D levels vary across organizations. According to the Endocrine Society Practice Guidelines on vitamin D, VDD is defined as a serum 25(OH)D < 20 ng/ml (i.e., 50 nmol/L), insufficiency as 21–29 ng/ml (i.e., 52.5–72.5 nmol/L), and sufficiency as at least 30 ng/ml (i.e., 75 nmol/L) for maximum musculoskeletal health [207]. These cut-offs have also been endorsed by other organizations such as the American Association for Clinical Endocrinologists, the American Geriatric Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation [208]. Whereas, according to the World Health Organization (WHO) and the current National Institute for Health and Clinical Care Excellence (NICE), UK guidelines, VDD is defined as a serum 25(OH)D < 10 ng/ml (i.e., 25 nmol/L) and insufficiency as 10–20 ng/ml (i.e., 25–50 nmol/L) [209].
More aggressive supplementation should be followed in the elderly and subjects with low exposure to sunshine (dark skinned people, people with poor exposure to sunlight due to cultural reasons, institutionalized patients) and poor nutrition. Despite general recommendations, clinicians should tailor vitamin D prescriptions accounting for several parameters, (obesity, nutritional status, diet, sunlight exposure) since one-size-fits-all recommendations of vitamin D supplementation are doomed to fail. For instance, it has been shown that patients with obesity require two to three times higher vitamin D supplementation to treat VDD [210, 211]. Even though toxicity from vitamin D is extremely rare, as with all treatments, moderation is safer than exaggeration. Interestingly the clinical utility of these cut-offs has been confirmed in large studies on mortality. Apart from the recent Mendelian randomization study showing higher mortality at 25(OH)D levels below 25 nmol/L, with the association plateauing at the deficiency cut-off level (50 nmol/L) [206], similar results were also yielded from the institute of medicine. In this report, a J-curve was shown in the relationship between mortality and blood levels of 25(OH)D, with a significant decline in mortality when 25(OH)D approached 30 ng/mL and then a slight increase that was apparent at 50 ng/mL [212]. However, some authors have argued that the increased mortality seen for 25(OH)D > 50 ng/ml, may be attributed to previous long-standing VDD for which subjects were treated [213].
Still, despite apparent optimal per os supplementation, many subjects do not achieve normal vitamin D levels. Predictive equations to guide vitamin D replacement doses have been formulated, such as the one by Singh et al., proposing a formula that accounts for initial vitamin D levels, age, BMI, serum albumin concentration, and desired change in vitamin D levels to estimate the optimal and personalized dose of vitamin D replacement needed [214]. Whether the application of this formula corrects vitamin D levels has not been confirmed in large-scale clinical studies.
6 Discussion
Despite the pleiotropic actions of vitamin D, most RCTs on the effect of vitamin D supplementation on improving a disease outcome have been negative. There are several inherent difficulties in studying the effects of vitamin D supplementation using standard RCT designs, as discussed in chapter 1.2. Still, several other considerations can be made. First, to be able to modify the course of a chronic disease, long follow-up is needed. This was the purpose of the recent large VIDA and VITAL RCTs [23, 176]. The mode of supplementation often varies, with some authors preferring intermittent bolus dosing and others daily dosing. Evidence suggests that intermittent bolus dosing (and generally extremely high dosing) should be avoided since it can even generate harmful events. Intermittent bolus dosing would also go against the ideal scenario in which there would be no fluctuations in the circulating levels of vitamin D, considering its multisystem homeostatic role. On the other hand larger doses (i.e., ~1000–2000 IU/day) should be preferred over too-small doses (i.e., ~400–800 IU/day) to expect any meaningful effect. Finally, it may be more appropriate in future investigations to compare subjects with high 25(OH)D vitamin levels with those not achieving normalization of vitamin D levels, rather than continue comparing groups based on the amount of vitamin D supplementation received.
Even though experts still debate the optimal cut-offs of 25(OH)D levels, it could be that these vary according to the disease of interest [215]. For instance, even though levels higher than 30 ng/ml are considered the target for maximum musculoskeletal health, it could be that this cut-off should be placed higher when vitamin D is given for its immunomodulation effects. Indeed, in the practice guidelines published in 2018 by Pludowski et al., 25(OH)D values in the range 30–50 ng/ml were recommended to achieve the pleiotropic actions of vitamin D and for optimal overall health [216]. Future studies should probably contemporaneously assess total and free 25(OH)D levels, as well as DBP and PTH values. This would be an important advancement in the planning of RCTs if we consider the studies by Carlberg and colleagues [217]. These investigators gave 0, 1600, or 3200 IU of vitamin D daily for 5 months to elderly prediabetic subjects. After assessing PTH response and other vitamin D biomarkers, they showed that 24% of their studied subjects were low responders, 51% mid responders, and 25% high responders [217], and similar rates were found also in healthy young individuals [218]. These studies set the groundwork, demonstrating that in humans in vivo, there is a spectrum of responsiveness to vitamin D supplementation, or a varying degree of vitamin D resistance.
7 Conclusions
The present narrative review provides an overview of the current evidence regarding the applications of vitamin D in a series of diseases. Despite the inherent difficulties in assessing the effects of vitamin D supplementation in RCTs, vitamin D supplementation has been shown to decrease acute respiratory infections, cancer mortality, and the incidence of T2D and autoimmune diseases. Moreover, subjects without obesity seem to benefit more from vitamin D supplementation, a finding that warrants further investigation. It also clearly emerges that VDD should be treated as it is associated with poor health outcomes and increased morbidity and mortality. However, vitamin D supplementation in vitamin D replete subjects does not seem to induce any clinically meaningful benefits. Considering that universal testing for vitamin D is not possible and is expensive, in everyday clinical practice it should be advisable to give vitamin D supplementation, which is cheap, well-tolerated, and easily available. In research settings, a holistic approach when studying the effects of vitamin D supplementation, such as evaluation of the whole vitamin D endocrine system, rather than only of 25(OH)D levels before and after treatment, the use of adequate and physiologic vitamin D dosing, controlling for the amount of vitamin D supplementation subjects on the placebo arms may receive, and sufficiently long follow-up are some aspects that need to be carefully considered in future studies.
Data Availability
Not applicable.
References
Naeem Z. Vitamin D deficiency—an ignored epidemic. Int J Health Sci (Qassim). 2010;4:V–VI.
Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord Germ. 2017;18:153–65.
Hoseinzadeh E, Taha P, Wei C, Godini H, Ashraf GM, Taghavi M, et al. The impact of air pollutants, UV exposure and geographic location on vitamin D deficiency. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc England. 2018;113:241–54.
Saternus R, Vogt T, Reichrath J. A critical appraisal of strategies to optimize vitamin D status in Germany, a population with a western diet. Nutrients. Switzerland. 2019;11:2682.
Grønborg IM, Tetens I, Christensen T, Andersen EW, Jakobsen J, Kiely M, et al. Vitamin D-fortified foods improve wintertime vitamin D status in women of Danish and Pakistani origin living in Denmark: a randomized controlled trial. Eur J Nutr Germ. 2020;59:741–53.
Jääskeläinen T, Itkonen ST, Lundqvist A, Erkkola M, Koskela T, Lakkala K, et al. The positive impact of general vitamin D food fortification policy on vitamin D status in a representative adult Finnish population: evidence from an 11-y follow-up based on standardized 25-hydroxyvitamin D data. Am J Clin Nutr United States. 2017;105:1512–20.
Pilz S, März W, Cashman KD, Kiely ME, Whiting SJ, Holick MF, et al. Rationale and plan for vitamin D food fortification: a review and guidance paper. Front Endocrinol (Lausanne). Switzerland; 2018;9:373.
Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr United States. 2008;88:582S-586S.
Tanaka Y, DeLuca HF. Stimulation of 1,25-dihydroxyvitamin D3 production by 1,25-dihydroxyvitamin D3 in the hypocalcaemic rat. Biochem J. 1983;214:893–7.
Bikle DD, Patzek S, Wang Y. Physiologic and pathophysiologic roles of extra renal CYP27b1: case report and review. Bone Rep United States. 2018;8:255–67.
Saponaro F, Saba A, Zucchi R. An update on vitamin D metabolism. Int J Mol Sci. Switzerland. 2020;21:6573.
Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol Off Publ Pan Am Soc Clin Virol Netherlands. 2011;50:194–200.
Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A, et al. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99:3373–81.
Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary reference intakes for calcium and vitamin D. In: Ross AC, Taylor CL, Yaktine AL, et al., editors. Washington (DC): National Academies Press; 2011.
Tsuprykov O, Chen X, Hocher C-F, Skoblo R, Yin L, Hocher B. Why should we measure free 25(OH) vitamin D? J Steroid Biochem Mol Biol England. 2018;180:87–104.
Yuan C, Shui IM, Wilson KM, Stampfer MJ, Mucci LA, Giovannucci EL. Circulating 25-hydroxyvitamin D, vitamin D binding protein and risk of advanced and lethal prostate cancer. Int J Cancer. 2019;144:2401–7.
Qi L, Ma W, Heianza Y, Zheng Y, Wang T, Sun D, et al. Independent and synergistic associations of biomarkers of vitamin D status with risk of coronary heart disease. Arterioscler Thromb Vasc Biol. 2017;37:2204–12.
Yu C, Xue H, Wang L, Chen Q, Chen X, Zhang Y, et al. Serum bioavailable and free 25-hydroxyvitamin D levels, but not its total level, are associated with the risk of mortality in patients with coronary artery disease. Circ Res United States. 2018;123:996–1007.
Norman AW, Nemere I, Zhou LX, Bishop JE, Lowe KE, Maiyar AC, et al. 1,25(OH)2-vitamin D3, a steroid hormone that produces biologic effects via both genomic and nongenomic pathways. J Steroid Biochem Mol Biol England. 1992;41:231–40.
Zmijewski MA, Carlberg C. Vitamin D receptor(s): in the nucleus but also at membranes? Exp Dermatol Den. 2020;29:876–84.
Boucher BJ. Why do so many trials of vitamin D supplementation fail? Endocr Connect England. 2020;9:R195-206.
Hahn J, Cook NR, Alexander EK, Friedman S, Walter J, Bubes V, et al. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ England. 2022;376:e066452.
LeBoff MS, Chou SH, Murata EM, Donlon CM, Cook NR, Mora S, et al. Effects of supplemental vitamin D on bone health outcomes in women and men in the VITamin D and OmegA-3 TriaL (VITAL). J Bone Miner Res Off J Am Soc Bone Miner Res. 2020;35:883–93.
Chalcraft JR, Cardinal LM, Wechsler PJ, Hollis BW, Gerow KG, Alexander BM, et al. Vitamin D synthesis following a single bout of sun exposure in older and younger men and women. Nutrients. Switzerland. 2020;12:2237.
Vranić L, Mikolašević I, Milić S. Vitamin D deficiency: consequence or cause of obesity? Medicina (Kaunas). Switzerland. 2019;55:541.
Bedner M, Lippa KA, Tai SS-C. An assessment of 25-hydroxyvitamin D measurements in comparability studies conducted by the Vitamin D Metabolites Quality Assurance Program. Clin Chim Acta. Netherlands. 2013;426:6–11.
Lappe JM, Heaney RP. Why randomized controlled trials of calcium and vitamin D sometimes fail. Dermatoendocrinol United States. 2012;4:95–100.
van Driel M, van Leeuwen JPTM. Vitamin D and bone: a story of endocrine and auto/paracrine action in osteoblasts. Nutrients. Switzerland. 2023;15:480.
Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology United States. 1999;140:4982–7.
Dardenne O, Prud’homme J, Hacking SA, Glorieux FH, St-Arnaud R. Correction of the abnormal mineral ion homeostasis with a high-calcium, high-phosphorus, high-lactose diet rescues the PDDR phenotype of mice deficient for the 25-hydroxyvitamin D-1alpha-hydroxylase (CYP27B1). Bone. United States; 2003;32:332–40.
Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, et al. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem United States. 2004;279:16754–66.
Bouillon R, Antonio L. Nutritional rickets: Historic overview and plan for worldwide eradication. J Steroid Biochem Mol Biol England. 2020;198:105563.
Scragg R. The vitamin D Assessment (ViDA) study—design and main findings. J Steroid Biochem Mol Biol England. 2020;198:105562.
Burt LA, Billington EO, Rose MS, Raymond DA, Hanley DA, Boyd SK. Effect of high-dose vitamin D supplementation on volumetric bone density and bone strength: a randomized clinical trial. JAMA. 2019;322:736–45.
Suda T, Takahashi N, Abe E. Role of vitamin D in bone resorption. J Cell Biochem United States. 1992;49:53–8.
Rodan GA, Martin TJ. Role of osteoblasts in hormonal control of bone resorption—a hypothesis. Calcif Tissue Int United States. 1981;33:349–51.
Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol England. 2018;6:847–58.
Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int J Establ Result Coop Betw Eur Found Osteoporos Natl Osteoporos Found USA. 2016;27:367–76.
Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819–29.
Bignotti B, Cadoni A, Martinoli C, Tagliafico A. Imaging of skeletal muscle in vitamin D deficiency. World J Radiol. 2014;6:119–24.
Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab United States. 2014;99:4336–45.
Scott D, Stuart AL, Kay D, Ebeling PR, Nicholson G, Sanders KM. Investigating the predictive ability of gait speed and quadriceps strength for incident falls in community-dwelling older women at high risk of fracture. Arch Gerontol Geriatr Netherlands. 2014;58:308–13.
Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM, et al. Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab United States. 2011;96:2997–3006.
Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, et al. Effect of vitamin D on falls: a meta-analysis. JAMA United States. 2004;291:1999–2006.
Guo J-L, Tsai Y-Y, Liao J-Y, Tu H-M, Huang C-M. Interventions to reduce the number of falls among older adults with/without cognitive impairment: an exploratory meta-analysis. Int J Geriatr Psychiatry England. 2014;29:661–9.
Kärkkäinen MK, Tuppurainen M, Salovaara K, Sandini L, Rikkonen T, Sirola J, et al. Does daily vitamin D 800 IU and calcium 1000 mg supplementation decrease the risk of falling in ambulatory women aged 65–71 years? A 3-year randomized population-based trial (OSTPRE-FPS). Maturitas Irel. 2010;65:359–65.
Glendenning P, Zhu K, Inderjeeth C, Howat P, Lewis JR, Prince RL. Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: a randomized controlled trial. J Bone Miner Res Off J Am Soc Bone Miner Res United States. 2012;27:170–6.
Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA United States. 2010;303:1815–22.
Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol England. 2014;2:307–20.
Bolland MJ, Grey A, Gamble GD, Reid IR. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol England. 2014;2:573–80.
Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, et al. 1,25-Dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem United States. 2007;282:29821–30.
Li YC. Molecular mechanism of vitamin D in the cardiovascular system. J Investig Med Off Publ Am Fed Clin Res. 2011;59:868–71.
Chen S, Sun Y, Agrawal DK. Vitamin D deficiency and essential hypertension. J Am Soc Hypertens. 2015;9:885–901.
Chen S, Gemelga G, Yeghiazarians Y. Is vitamin D supplementation an effective treatment for hypertension? Curr Hypertens Rep. United States. 2022.
Bernini G, Carrara D, Bacca A, Carli V, Virdis A, Rugani I, et al. Effect of acute and chronic vitamin D administration on systemic renin angiotensin system in essential hypertensives and controls. J Endocrinol Investig Italy. 2013;36:216–20.
Forman JP, Scott JB, Ng K, Drake BF, Suarez EG, Hayden DL, et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertens (Dallas, Tex 1979). 2013;61:779–85.
Bricio-Barrios JAR, Palacios-Fonseca AJMS, Del Toro-Equihua M, Sanchez-Ramirez CA. Effect of calcitriol supplementation on blood pressure in older adults. J Nutr Gerontol Geriatr. United States. 2016;35:243–52.
Sheikh V, Mozaianimonfared A, Gharakhani M, Poorolajal J, Ph D. Effect of vitamin D supplementation versus placebo on essential hypertension in patients with vitamin D deficiency: a double-blind randomized clinical trial. J Clin Hypertens (Greenwich). 2020;22:1867–73.
Witham MD, Ireland S, Houston JG, Gandy SJ, Waugh S, Macdonald TM, et al. Vitamin D therapy to reduce blood pressure and left ventricular hypertrophy in resistant hypertension: randomized, controlled trial. Hypertens (Dallas, Tex 1979) United States. 2014;63:706–12.
Wang J, Zhou JJ, Robertson GR, Lee VW. Vitamin D in vascular calcification: a double-edged sword? Nutrients. 2018;10:652.
Jorde R, Sneve M, Torjesen P, Figenschau Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med England. 2010;267:462–72.
Carrara D, Bernini M, Bacca A, Rugani I, Duranti E, Virdis A, et al. Cholecalciferol administration blunts the systemic renin-angiotensin system in essential hypertensives with hypovitaminosis D. J Renin Angiotensin Aldosterone Syst England. 2014;15:82–7.
Manson JE, Cook NR, Lee I-M, Christen W, Bassuk SS, Mora S, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33–44.
Bischoff-Ferrari HA, Vellas B, Rizzoli R, Kressig RW, da Silva JAP, Blauth M, et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA. 2020;324:1855–68.
Challoumas D, Stavrou A, Pericleous A, Dimitrakakis G. Effects of combined vitamin D–calcium supplements on the cardiovascular system: should we be cautious? Atherosclerosis Ireland. 2015;238:388–98.
Davies MR, Hruska KA. Pathophysiological mechanisms of vascular calcification in end-stage renal disease. Kidney Int United States. 2001;60:472–9.
Carvalho LSF, Sposito AC. Vitamin D for the prevention of cardiovascular disease: are we ready for that? Atherosclerosis Ireland. 2015;241:729–40.
Wang J-H, Keisala T, Solakivi T, Minasyan A, Kalueff AV, Tuohimaa P. Serum cholesterol and expression of ApoAI, LXRbeta and SREBP2 in vitamin D receptor knock-out mice. J Steroid Biochem Mol Biol England. 2009;113:222–6.
Christensen R, Lorenzen JK, Svith CR, Bartels EM, Melanson EL, Saris WH, et al. Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obes Rev Off J Int Assoc Study Obes England. 2009;10:475–86.
Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348: g1903.
Schöttker B, Jorde R, Peasey A, Thorand B, Jansen EHJM, de Groot L, et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348: g3656.
Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ England. 2016;355:i5953.
Zhang P, Guo D, Xu B, Huang C, Yang S, Wang W, et al. Association of serum 25-hydroxyvitamin D with cardiovascular outcomes and all-cause mortality in individuals with prediabetes and diabetes: results from the UK biobank prospective cohort study. Diabetes Care United States. 2022;45:1219–29.
Sluyter JD, Camargo CAJ, Stewart AW, Waayer D, Lawes CMM, Toop L, et al. Effect of monthly, high-dose, long-term vitamin D Supplementation on central blood pressure parameters: a randomized controlled trial substudy. J Am Heart Assoc. 2017;6:e006802.
Barbarawi M, Kheiri B, Zayed Y, Barbarawi O, Dhillon H, Swaid B, et al. Vitamin D supplementation and cardiovascular disease risks in more than 83 000 individuals in 21 randomized clinical trials: a meta-analysis. JAMA Cardiol. 2019;4:765–76.
Wang L, Manson JE, Song Y, Sesso HD. Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med United States. 2010;152:315–23.
Mao P-J, Zhang C, Tang L, Xian Y-Q, Li Y-S, Wang W-D, et al. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol Netherlands. 2013;169:106–11.
Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745–54.
Mirhosseini N, Rainsbury J, Kimball SM. Vitamin D supplementation, serum 25(OH)D concentrations and cardiovascular disease risk factors: a systematic review and meta-analysis. Front Cardiovasc Med. 2018;5:87.
Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res Off J Am Soc Bone Miner Res United States. 2006;21:37–47.
L Bishop E, Ismailova A, Dimeloe S, Hewison M, White JH. Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory. JBMR Plus. England. 2021;5:e10405.
Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab United States. 2001;86:888–94.
Szymczak I, Pawliczak R. The active metabolite of vitamin D3 as a potential immunomodulator. Scand J Immunol England. 2016;83:83–91.
Helming L, Böse J, Ehrchen J, Schiebe S, Frahm T, Geffers R, et al. 1alpha,25-dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood United States. 2005;106:4351–8.
Sundaram ME, Coleman LA. Vitamin D and influenza. Adv Nutr. 2012;3:517–25.
Bruce D, Ooi JH, Yu S, Cantorna MT. Vitamin D and host resistance to infection? Putting the cart in front of the horse. Exp Biol Med (Maywood). 2010;235:921–7.
Khare D, Godbole NM, Pawar SD, Mohan V, Pandey G, Gupta S, et al. Calcitriol [1, 25[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur J Nutr Germ. 2013;52:1405–15.
Berry DJ, Hesketh K, Power C, Hyppönen E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr England. 2011;106:1433–40.
Aregbesola A, Voutilainen S, Nurmi T, Virtanen JK, Ronkainen K, Tuomainen T-P. Serum 25-hydroxyvitamin D3 and the risk of pneumonia in an ageing general population. J Epidemiol Community Health England. 2013;67:533–6.
Mamani M, Muceli N, Ghasemi Basir HR, Vasheghani M, Poorolajal J. Association between serum concentration of 25-hydroxyvitamin D and community-acquired pneumonia: a case-control study. Int J Gen Med. 2017;10:423–9.
Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr United States. 2010;91:1255–60.
Li-Ng M, Aloia JF, Pollack S, Cunha BA, Mikhail M, Yeh J, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect England. 2009;137:1396–404.
Jorde R, Witham M, Janssens W, Rolighed L, Borchhardt K, de Boer IH, et al. Vitamin D supplementation did not prevent influenza-like illness as diagnosed retrospectively by questionnaires in subjects participating in randomized clinical trials. Scand J Infect Dis. 2012;44:126–32.
Urashima M, Mezawa H, Noya M, Camargo CAJ. Effects of vitamin D supplements on influenza A illness during the 2009 H1N1 pandemic: a randomized controlled trial. Food Funct England. 2014;5:2365–70.
Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356: i6583.
Jolliffe DA, Camargo CAJ, Sluyter JD, Aglipay M, Aloia JF, Ganmaa D, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol England. 2021;9:276–92.
Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol England. 2007;103:793–8.
Rook GA, Steele J, Fraher L, Barker S, Karmali R, O’Riordan J, et al. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57:159–63.
Crowle AJ, Ross EJ, May MH. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1987;55:2945–50.
Cao Y, Wang X, Liu P, Su Y, Yu H, Du J. Vitamin D and the risk of latent tuberculosis infection: a systematic review and meta-analysis. BMC Pulm Med. 2022;22:39.
Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med United States. 2007;176:208–13.
Ganmaa D, Uyanga B, Zhou X, Gantsetseg G, Delgerekh B, Enkhmaa D, et al. Vitamin D supplements for prevention of tuberculosis infection and disease. N Engl J Med. 2020;383:359–68.
Wu H-X, Xiong X-F, Zhu M, Wei J, Zhuo K-Q, Cheng D-Y. Effects of vitamin D supplementation on the outcomes of patients with pulmonary tuberculosis: a systematic review and meta-analysis. BMC Pulm Med. 2018;18:108.
Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, et al. Mechanisms in endocrinology: vitamin D and COVID-19. Eur J Endocrinol England. 2020;183:R133–47.
Charoenngam N, Shirvani A, Holick MF. Vitamin D and its potential benefit for the COVID-19 pandemic. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2021;27:484–93.
Katz J, Yue S, Xue W. Increased risk for COVID-19 in patients with vitamin D deficiency. Nutrition. United States; 2021;84:111106.
Jude EB, Ling SF, Allcock R, Yeap BXY, Pappachan JM. Vitamin D deficiency is associated with higher hospitalization risk from COVID-19: a retrospective case-control study. J Clin Endocrinol Metab United States. 2021;106:e4708–15.
Annweiler G, Corvaisier M, Gautier J, Dubée V, Legrand E, Sacco G, et al. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients. 2020;12:3377.
Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203: 105751.
Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325:1053–60.
Tentolouris N, Samakidou G, Eleftheriadou I, Tentolouris A, Jude EB. The effect of vitamin D supplementation on mortality and intensive care unit admission of COVID-19 patients. A systematic review, meta-analysis and meta-regression. Diabetes Metab Res Rev. 2022;38:e3517.
Shah K, Saxena D, Mavalankar D. Vitamin D supplementation, COVID-19 and disease severity: a meta-analysis. QJM. 2021;114:175–81.
Pal R, Banerjee M, Bhadada SK, Shetty AJ, Singh B, Vyas A. Vitamin D supplementation and clinical outcomes in COVID-19: a systematic review and meta-analysis. J Endocrinol Investig. 2022;45:53–68.
Jolliffe DA, Holt H, Greenig M, Talaei M, Perdek N, Pfeffer P, et al. Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and covid-19: phase 3 randomised controlled trial (CORONAVIT). BMJ. England. 2022;378:e071230.
Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin D and diabetes. Endocrinol Metab Clin N Am United States. 2010;39:419–46 (table of contents).
Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology United States. 1986;119:84–90.
Szymczak-Pajor I, Drzewoski J, Śliwińska A. The molecular mechanisms by which vitamin D prevents insulin resistance and associated disorders. Int J Mol Sci. Switzerland. 2020;21:6644.
Maestro B, Campión J, Dávila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J Jpn. 2000;47:383–91.
Grimnes G, Figenschau Y, Almås B, Jorde R, Vitamin D. insulin secretion, sensitivity, and lipids: results from a case-control study and a randomized controlled trial using hyperglycemic clamp technique. Diabetes. 2011;60:2748–57.
Mitchell DM, Leder BZ, Cagliero E, Mendoza N, Henao MP, Hayden DL, et al. Insulin secretion and sensitivity in healthy adults with low vitamin D are not affected by high-dose ergocalciferol administration: a randomized controlled trial. Am J Clin Nutr. 2015;102:385–92.
Jamka M, Woźniewicz M, Jeszka J, Mardas M, Bogdański P, Stelmach-Mardas M. The effect of vitamin D supplementation on insulin and glucose metabolism in overweight and obese individuals: systematic review with meta-analysis. Sci Rep England. 2015;5:16142.
Rebelos E, Mari A, Oikonen V, Iida H, Nuutila P, Ferrannini E. Evaluation of renal glucose uptake with [(18)F]FDG-PET: methodological advancements and metabolic outcomes. Metabolism. United States; 2023;141:155382.
Rebelos E, Bucci M, Karjalainen T, Oikonen V, Alessandra B, Hannukainen JC, et al. Insulin resistance is associated with enhanced brain glucose uptake during euglycemic hyperinsulinemia: a large-scale PET cohort. Diabetes Care. 2021;44:1–7.
Dadson P, Landini L, Helmiö M, Hannukainen JC, Immonen H, Honka MJ, et al. Effect of bariatric surgery on adipose tissue glucose metabolism in different depots in patients with or without type 2 diabetes. Diabetes Care. 2016;39:292–9.
Immonen H, Hannukainen JC, Iozzo P, Soinio M, Salminen P, Saunavaara V, et al. Effect of bariatric surgery on liver glucose metabolism in morbidly obese diabetic and non-diabetic patients. J Hepatol Netherlands. 2014;60:377–83.
Need AG, O’Loughlin PD, Horowitz M, Nordin BEC. Relationship between fasting serum glucose, age, body mass index and serum 25 hydroxyvitamin D in postmenopausal women. Clin Endocrinol (Oxf) England. 2005;62:738–41.
Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990–2000. Diabetes. 2008;57:2619–25.
Hyppönen E, Power C. Vitamin D status and glucose homeostasis in the 1958 British birth cohort: the role of obesity. Diabetes Care United States. 2006;29:2244–6.
Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care United States. 2004;27:2813–8.
Dawson-Hughes B, Staten MA, Knowler WC, Nelson J, Vickery EM, LeBlanc ES, et al. Intratrial exposure to vitamin d and new-onset diabetes among adults with prediabetes: a secondary analysis from the vitamin D and type 2 diabetes (D2d) study. Diabetes Care. 2020;43:2916–22.
Barbarawi M, Zayed Y, Barbarawi O, Bala A, Alabdouh A, Gakhal I, et al. Effect of vitamin D supplementation on the incidence of diabetes mellitus. J Clin Endocrinol Metab. United States. 2020;105:dgaa335.
Zhang Y, Tan H, Tang J, Li J, Chong W, Hai Y, et al. Effects of vitamin D supplementation on prevention of type 2 diabetes in patients with prediabetes: a systematic review and meta-analysis. Diabetes Care United States. 2020;43:1650–8.
Wu C, Qiu S, Zhu X, Li L. Vitamin D supplementation and glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Metabolism United States. 2017;73:67–76.
Li X, Liu Y, Zheng Y, Wang P, Zhang Y. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Nutrients. 2018;10:375.
Poel YHM, Hummel P, Lips P, Stam F, van der Ploeg T, Simsek S. Vitamin D and gestational diabetes: a systematic review and meta-analysis. Eur J Intern Med Netherlands. 2012;23:465–9.
Irwinda R, Hiksas R, Lokeswara AW, Wibowo N. Vitamin D supplementation higher than 2000 IU/day compared to lower dose on maternal-fetal outcome: systematic review and meta-analysis. Womens Health (Lond Engl). United States; 2022;18:17455057221111066.
Faye PA, Poumeaud F, Miressi F, Lia AS, Demiot C, Magy L, et al. Focus on 1,25-dihydroxyvitamin D3 in the peripheral nervous system. Front Neurosci. Switzerland; 2019;13:348.
Tague SE, Smith PG. Vitamin D receptor and enzyme expression in dorsal root ganglia of adult female rats: modulation by ovarian hormones. J Chem Neuroanat Netherlands. 2011;41:1–12.
Shehab D, Al-Jarallah K, Abdella N, Mojiminiyi OA, Al MH. Prospective evaluation of the effect of short-term oral vitamin d supplementation on peripheral neuropathy in type 2 diabetes mellitus. Med Princ Pract Int J Kuwait Univ Heal Sci Cent Switzerland. 2015;24:250–6.
Costa PLF, França MM, Katayama ML, Carneiro ET, Martin RM, Folgueira MAK, et al. Transcriptomic response to 1,25-dihydroxyvitamin D in human fibroblasts with or without a functional vitamin D receptor (VDR): novel target genes and insights into VDR basal transcriptional activity. Cells. 2019;8:318.
Ding J, Kwan P, Ma Z, Iwashina T, Wang J, Shankowsky HA, et al. Synergistic effect of vitamin D and low concentration of transforming growth factor beta 1, a potential role in dermal wound healing. Burns Netherlands. 2016;42:1277–86.
Dobak J, Grzybowski J, Liu FT, Landon B, Dobke M. 1,25-Dihydroxyvitamin D3 increases collagen production in dermal fibroblasts. J Dermatol Sci Netherlands. 1994;8:18–24.
Gonzalez-Curiel I, Trujillo V, Montoya-Rosales A, Rincon K, Rivas-Calderon B, deHaro-Acosta J, et al. 1,25-dihydroxyvitamin D3 induces LL-37 and HBD-2 production in keratinocytes from diabetic foot ulcers promoting wound healing: an in vitro model. PLoS ONE. 2014;9: e111355.
Tian XQ, Chen TC, Holick MF. 1,25-dihydroxyvitamin D3: a novel agent for enhancing wound healing. J Cell Biochem United States. 1995;59:53–6.
Trujillo V, Marín-Luevano P, González-Curiel I, Rodríguez-Carlos A, Ramírez-Reyes M, Layseca-Espinosa E, et al. Calcitriol promotes proangiogenic molecules in keratinocytes in a diabetic foot ulcer model. J Steroid Biochem Mol Biol England. 2017;174:303–11.
Alam U, Petropoulos IN, Ponirakis G, Ferdousi M, Asghar O, Jeziorska M, et al. Vitamin D deficiency is associated with painful diabetic neuropathy. Diabetes Metab Res Rev England. 2021;37:e3361.
Zubair M, Malik A, Meerza D, Ahmad J. 25-Hydroxyvitamin D [25(OH)D] levels and diabetic foot ulcer: is there any relationship? Diabetes Metab Syndr Netherlands. 2013;7:148–53.
Tiwari S, Pratyush DD, Gupta SK, Singh SK. Vitamin D deficiency is associated with inflammatory cytokine concentrations in patients with diabetic foot infection. Br J Nutr England. 2014;112:1938–43.
Dai J, Jiang C, Chen H, Chai Y. Vitamin D and diabetic foot ulcer: a systematic review and meta-analysis. Nutr Diabetes. 2019;9:8.
Yammine K, Hayek F, Assi C. Is there an association between vitamin D and diabetic foot disease? A meta-analysis. Wound Repair Regener Off Publ Wound Heal Soc Eur Tissue Repair Soc. United States. 2020;28:90–6.
Razzaghi R, Pourbagheri H, Momen-Heravi M, Bahmani F, Shadi J, Soleimani Z, et al. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. J Diabetes Complic United States. 2017;31:766–72.
Halschou-Jensen PM, Sauer J, Bouchelouche P, Fabrin J, Brorson S, Ohrt-Nissen S. Improved healing of diabetic foot ulcers after high-dose vitamin D: a randomized double-blinded clinical trial. Int J Low Extrem Wounds. United States. 2021;15347346211020268.
Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat Netherlands. 2005;29:21–30.
Gezen-Ak D, Dursun E, Yilmazer S. Vitamin D inquiry in hippocampal neurons: consequences of vitamin D-VDR pathway disruption on calcium channel and the vitamin D requirement. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol Italy. 2013;34:1453–8.
Cui X, Eyles DW. Vitamin D and the central nervous system: causative and preventative mechanisms in brain disorders. Nutrients. Switzerland. 2022;14:4353.
Menéndez SG, Martín Giménez VM, Holick MF, Barrantes FJ, Manucha W. COVID-19 and neurological sequelae: vitamin D as a possible neuroprotective and/or neuroreparative agent. Life Sci Netherlands. 2022;297:120464.
AlJohri R, AlOkail M, Haq SH. Neuroprotective role of vitamin D in primary neuronal cortical culture. eNeurologicalSci. Netherlands. 2019;14:43–8.
Mizwicki MT, Liu G, Fiala M, Magpantay L, Sayre J, Siani A, et al. 1α,25-dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-β phagocytosis and inflammation in Alzheimer’s disease patients. J Alzheimers Dis. 2013;34:155–70.
Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Sääksjärvi K, Heliövaara M. Serum vitamin D and the risk of Parkinson disease. Arch Neurol. 2010;67:808–11.
Shrestha S, Lutsey PL, Alonso A, Huang X, Mosley THJ, Chen H. Serum 25-hydroxyvitamin D concentrations in mid-adulthood and Parkinson’s disease risk. Mov Disord. 2016;31:972–8.
Chitsaz A, Maracy M, Basiri K, Izadi Boroujeni M, Tanhaei AP, Rahimi M, et al. 25-hydroxyvitamin d and severity of Parkinson’s disease. Int J Endocrinol. 2013;2013: 689149.
The Parkinson progression marker initiative (PPMI). Prog Neurobiol. 2011;95:629–35.
Hiller AL, Murchison CF, Lobb BM, O’Connor S, O’Connor M, Quinn JF. A randomized, controlled pilot study of the effects of vitamin D supplementation on balance in Parkinson’s disease: Does age matter? PLoS ONE. 2018;13: e0203637.
Peterson A, Mattek N, Clemons A, Bowman GL, Buracchio T, Kaye J, et al. Serum vitamin D concentrations are associated with falling and cognitive function in older adults. J Nutr Health Aging. 2012;16:898–901.
Anastasiou CA, Yannakoulia M, Scarmeas N. Vitamin D and cognition: an update of the current evidence. J Alzheimers Dis Netherlands. 2014;42(Suppl 3):S71-80.
Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, et al. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1981;78:4990–4.
Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer England. 2007;7:684–700.
Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer England. 2014;14:342–57.
Estébanez N, Gómez-Acebo I, Palazuelos C, Llorca J, Dierssen-Sotos T. Vitamin D exposure and risk of breast cancer: a meta-analysis. Sci Rep. 2018;8:9039.
Maalmi H, Walter V, Jansen L, Boakye D, Schöttker B, Hoffmeister M, et al. Association between blood 25-hydroxyvitamin D levels and survival in colorectal cancer patients: an updated systematic review and meta-analysis. Nutrients. 2018;10:896.
Song Z-Y, Yao Q, Zhuo Z, Ma Z, Chen G. Circulating vitamin D level and mortality in prostate cancer patients: a dose-response meta-analysis. Endocr Connect. 2018;7:R294-303.
Wang W, Li G, He X, Gao J, Wang R, Wang Y, et al. Serum 25-hydroxyvitamin D levels and prognosis in hematological malignancies: a systematic review and meta-analysis. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol Germ. 2015;35:1999–2005.
Brunner RL, Wactawski-Wende J, Caan BJ, Cochrane BB, Chlebowski RT, Gass MLS, et al. The effect of calcium plus vitamin D on risk for invasive cancer: results of the Women’s Health Initiative (WHI) calcium plus vitamin D randomized clinical trial. Nutr Cancer. 2011;63:827–41.
Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial). J Clin Endocrinol Metab United States. 2012;97:614–22.
Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469.
Scragg R, Khaw K-T, Toop L, Sluyter J, Lawes CMM, Waayer D, et al. Monthly high-dose vitamin d supplementation and cancer risk: a post hoc analysis of the vitamin D assessment randomized clinical trial. JAMA Oncol. 2018;4: e182178.
Keum N, Lee DH, Greenwood DC, Manson JE, Giovannucci E. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann Oncol Off J Eur Soc Med Oncol. 2019;30:733–43.
Li S, Jin Y, Fu W, Cox AD, Lee D, Reddivari L. Intermittent antibiotic treatment accelerated the development of colitis in IL-10 knockout mice. Biomed Pharmacother France. 2022;146:112486.
Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell United States. 1993;75:263–74.
Liu M, Yuan W, Park S. Association between IL-10 rs3024505 and susceptibility to inflammatory bowel disease: a systematic review and meta-analysis. Cytokine England. 2022;149:155721.
Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr United States. 2000;130:2648–52.
Lim W-C, Hanauer SB, Li YC. Mechanisms of disease: vitamin D and inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol England. 2005;2:308–15.
Raftery T, Martineau AR, Greiller CL, Ghosh S, McNamara D, Bennett K, et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: results from a randomised double-blind placebo-controlled study. United Eur Gastroenterol J. 2015;3:294–302.
Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, et al. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012;12:57.
Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F. Association between inflammatory bowel disease and vitamin D deficiency: a systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:2708–17.
Gubatan J, Chou ND, Nielsen OH, Moss AC. Systematic review with meta-analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther England. 2019;50:1146–58.
Narula N, Cooray M, Anglin R, Muqtadir Z, Narula A, Marshall JK. Impact of high-dose vitamin D3 supplementation in patients with Crohn’s disease in remission: a pilot randomized double-blind controlled study. Dig Dis Sci United States. 2017;62:448–55.
de Bruyn JR, Bossuyt P, Ferrante M, West RL, Dijkstra G, Witteman BJ, et al. High-dose vitamin D does not prevent postoperative recurrence of Crohn’s Disease in a randomized placebo-controlled trial. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc United States. 2021;19:1573–1582.e5.
Murdaca G, Tonacci A, Negrini S, Greco M, Borro M, Puppo F, et al. Emerging role of vitamin D in autoimmune diseases: an update on evidence and therapeutic implications. Autoimmun Rev Netherlands. 2019;18:102350.
May E, Asadullah K, Zügel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy Netherlands. 2004;3:377–93.
Slominski AT, Kim T-K, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, et al. RORα and ROR γ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB. 2014;28:2775–89.
Slominski AT, Kim T-K, Hobrath JV, Oak ASW, Tang EKY, Tieu EW, et al. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORα and RORγ. J Steroid Biochem Mol Biol. 2017;173:42–56.
Lemke D, Klement RJ, Schweiger F, Schweiger B, Spitz J. Vitamin D resistance as a possible cause of autoimmune diseases: a hypothesis confirmed by a therapeutic high-dose vitamin D protocol. Front Immunol. 2021;12: 655739.
Harrison SR, Li D, Jeffery LE, Raza K, Hewison M. Vitamin D, autoimmune disease and rheumatoid arthritis. Calcif Tissue Int United States. 2020;106:58–75.
Filoni A, Vestita M, Congedo M, Giudice G, Tafuri S, Bonamonte D. Association between psoriasis and vitamin D: duration of disease correlates with decreased vitamin D serum levels: an observational case-control study. Medicine (Baltimore). United States. 2018;97:e11185.
Kurtul BE, Özer PA, Aydinli MS. The association of vitamin D deficiency with tear break-up time and Schirmer testing in non-Sjögren dry eye. Eye (Lond) England. 2015;29:1081–4.
Vieira IH, Rodrigues D, Paiva I. Vitamin D and autoimmune thyroid disease-cause, consequence, or a vicious cycle? Nutrients. Switzerland. 2020;12:2791.
Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet. United States. 2009;41:824–8.
Jacobs BM, Noyce AJ, Giovannoni G, Dobson R. BMI and low vitamin D are causal factors for multiple sclerosis: a Mendelian randomization study. Neurol Neuroimmunol Neuroinflamm. 2020;7:e662.
Rhead B, Bäärnhielm M, Gianfrancesco M, Mok A, Shao X, Quach H, et al. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol Genet. 2016;2: e97.
Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA United States. 2006;296:2832–8.
Duan S, Lv Z, Fan X, Wang L, Han F, Wang H, et al. Vitamin D status and the risk of multiple sclerosis: a systematic review and meta-analysis. Neurosci Lett Irel. 2014;570:108–13.
Finamor DC, Sinigaglia-Coimbra R, Neves LCM, Gutierrez M, Silva JJ, Torres LD, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinology. 2013;5:222–34.
McLaughlin L, Clarke L, Khalilidehkordi E, Butzkueven H, Taylor B, Broadley SA. Vitamin D for the treatment of multiple sclerosis: a meta-analysis. J Neurol Germ. 2018;265:2893–905.
Bouillon R, Manousaki D, Rosen C, Trajanoska K, Rivadeneira F, Richards JB. The health effects of vitamin D supplementation: evidence from human studies. Nat Rev Endocrinol. 2022;18:96–110.
Sutherland JP, Zhou A, Hyppönen E. Vitamin D deficiency increases mortality risk in the UK biobank: a nonlinear mendelian randomization study. Ann Intern Med United States. 2022;175:1552–9.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab United States. 2011;96:1911–30.
Recommendations abstracted from the American Geriatrics Society consensus statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. United States. 2014;62:147–52.
Excellence NI for H and CC (NICE). Vitamin D deficiency in adults - treatment and prevention. https//cks.nice.org.uk/topics/vitamin-d-deficiency-inadults-Treat. Accessed 10 Aug 2022
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr United States. 2000;72:690–3.
Boonchaya-anant P, Holick MF, Apovian CM. Serum 25-hydroxyvitamin D levels and metabolic health status in extremely obese individuals. Obesity (Silver Spring) United States. 2014;22:2539–43.
Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary reference intakes for calcium and vitamin D. Washington DC: The National Academies Press; 2011.
Kroll MH, Bi C, Garber CC, Kaufman HW, Liu D, Caston-Balderrama A, et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS ONE. 2015;10: e0118108.
Singh G, Bonham AJ. A predictive equation to guide vitamin D replacement dose in patients. J Am Board Fam Med United States. 2014;27:495–509.
Grant WB, Al Anouti F, Boucher BJ, Dursun E, Gezen-Ak D, Jude EB, et al. A narrative review of the evidence for variations in serum 25-hydroxyvitamin D concentration thresholds for optimal health. Nutrients. Switzerland. 2022;14:639.
Pludowski P, Holick MF, Grant WB, Konstantynowicz J, Mascarenhas MR, Haq A, et al. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol England. 2018;175:125–35.
Saksa N, Neme A, Ryynänen J, Uusitupa M, de Mello VDF, Voutilainen S, et al. Dissecting high from low responders in a vitamin D3 intervention study. J Steroid Biochem Mol Biol England. 2015;148:275–82.
Seuter S, Virtanen JK, Nurmi T, Pihlajamäki J, Mursu J, Voutilainen S, et al. Molecular evaluation of vitamin D responsiveness of healthy young adults. J Steroid Biochem Mol Biol England. 2017;174:314–21.
Funding
None.
Author information
Authors and Affiliations
Contributions
ER and EJ searched the literature and drafted the manuscript. NT revised critically the text. All authors approved the final version of the text.
Corresponding author
Ethics declarations
Conflict of interest
Edward Jude, Eleni Rebelos and Nikolaos Tentolouris declare that they have no conflicts of interest.
Ethical approval
Not applicable
Informed consent
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rebelos, E., Tentolouris, N. & Jude, E. The Role of Vitamin D in Health and Disease: A Narrative Review on the Mechanisms Linking Vitamin D with Disease and the Effects of Supplementation. Drugs 83, 665–685 (2023). https://doi.org/10.1007/s40265-023-01875-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01875-8