Abstract
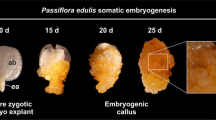
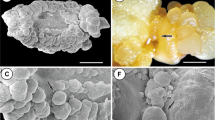
The characterization of cellular changes that occur during somatic embryogenesis is essential for understanding the factors involved in the transition of somatic cells into embryogenically competent cells and determination of cells and/or tissues involved. The present study describes the anatomical and ultrastructural events that lead to the formation of somatic embryos in the model system of the wild passion fruit (Passiflora cincinnata). Mature zygotic embryos were inoculated in Murashige and Skoog induction media supplemented with 2,4-dichlorophenoxyacetic acid and 6-benzyladenine. Zygotic embryo explants at different development stages were collected and processed by conventional methods for studies using light, scanning, and transmission electron microscopy (TEM). Histochemical tests were used to examine the mobilization of reserves. The differentiation of the somatic embryos began in the abaxial side of the cotyledon region. Protuberances were formed from the meristematic proliferation of the epidermal and mesophyll cells. These cells had large nuclei, dense cytoplasm with a predominance of mitochondria, and a few reserve compounds. The protuberances extended throughout the abaxial surface of the cotyledons. The ongoing differentiation of peripheral cells of these structures led to the formation of proembryogenic zones, which, in turn, dedifferentiated into somatic embryos of multicellular origin. In the initial stages of embryogenesis, the epidermal and mesophyll cells showed starch grains and less lipids and protein reserves than the starting explant. These results provide detailed information on anatomical and ultrastructural changes involved in the acquisition of embryogenic competence and embryo differentiation that has been lacking so far in Passiflora.




Similar content being viewed by others
References
Alemanno L, Berthouly M, Michaux-Ferrière N (1996) Histology of somatic embryogenesis from floral tissues cocoa. Plant Cell Tiss Organ Cult 46:187–194. doi:10.1007/BF02307094
Alexandre RS, Otoni WC, Dias JMM, Bruckner CH, Lopes JC (2009) In vitro propagation of passionfruit. In: Alexandre RS, Bruckner CH, Lopes JC (eds) Propagation of passionfruit: morphological, physiological and genetic aspects. EDUFES, Vitória, pp 117–184, in Portuguese
Alfenas PF, Braz ASK, Torres LB, Santana EN, Nascimento AVS, Carvalho MG, Otoni WC, Zerbini FM (2005) Transgenic passionfruit expressing RNA derived from Cowpea aphid-borne mosaic virus is resistant to passionfruit woodiness disease. Fitopat Bras 30:33–38. doi:10.1590/S0100-41582005000100005
Appezzato-da-Glória B, Machado SR (2004) Ultrastructural analysis of in vitro direct and indirect organogenesis. Rev Bras Bot 27:429–437. doi:10.1590/S0100-84042004000300004
Appezzato-da-Glória B, Vieira MLC, Dornelas MC (1999) Anatomical studies of in vitro organogenesis induced in leaf-derived explants of passionfruit. Pesq Agropec Bras 34:2007–2013. doi:10.1590/S0100-204X1999001100005
Appezzato-da-Glória B, Fernando JA, Machado SR, Vieira MLC (2005) Morphological, anatomical, histochemical and ultrastructural studies of in vitro organogenesis of passionfruit. In: Faleiro FG, Junqueira NTV, Braga MF (eds) Passionfruit: germplasm and breeding, 1st edn. Embrapa Cerrados, Planaltina, pp 387–407, in Portuguese
Barciela J, Vieitez AM (1993) Anatomical sequence and morphometric analysis during somatic embryogenesis on cultured cotyledon explants of Camellia japonica L. Ann Bot 71:395–404. doi:10.1006/anbo.1993.1050
Bewley JD, Black M (1994) Seeds: physiology of development and germination. Plenum, London
Branca C, Torelli A, Fermi P, Altamura MM, Bassi M (1994) Early phases in in vitro culture of tomato cotyledons: starch accumulation and protein pattern in relation to the hormonal treatment. Protoplasma 182:59–64. doi:10.1007/BF01403689
Buckeridge MS, Aidar MPM, Santos HP, Tiné MAS (2004) Acúmulo de reservas. In: Ferreira AG, Borghetti F (eds) Germination: of the basic to the applied, 1st edn. ARTMED, Porto Alegre, pp 31–50, in Portuguese
Cangahuala-Inocente GC, Steiner N, Santos M, Guerra MP (2004) Morphological analysis and histochemistry of Feijoa sellowiana somatic embryogenesis. Protoplasma 224:33–40. doi:10.1007/s00709-004-0055-5
Cangahuala-Inocente GC, Steiner N, Maldonado SB, Guerra MP (2009) Patterns of protein and carbohydrate accumulation during somatic embryogenesis of Acca sellowiana. Pesq Agropec Bras 44:217–224. doi:10.1590/S0100-204X2009000300001
Canhoto JM, Mesquita JF, Cruz GS (1996) Ultrastructural changes in cotyledons of pineapple guava (Myrtaceae) during somatic embryogenesis. Ann Bot 78:513–521. doi:10.1006/anbo.1996.0149
Dias LLC, Santa-Catarina C, Ribeiro DM, Barros RS, Floh EIS, Otoni WC (2009) Ethylene and polyamine production patterns during in vitro shoot organogenesis of two passion fruit species as affected by polyamines and their inhibitor. Plant Cell Tiss Organ Cult 99:199–208. doi:10.1007/s11240-009-9594-y
Dias LLC, Ribeiro DM, Santa-Catarina C, Barros RS, Floh EIS, Otoni WC (2010) Ethylene and polyamine interactions in morphogenesis of Passiflora cincinnata: effects of ethylene biosynthesis and action modulators, as well as ethylene scavengers. Plant Growth Reg 62:9–19. doi:10.1007/s10725-010-9478-5
Dornelas MC, Vieira MLC (1994) Tissue culture studies on species of Passiflora. Plant Cell Tiss Organ Cult 36:211–217. doi:10.1007/BF00037722
Drew RA (1997) Micropropagation of Passiflora species (passion fruit). In: Bajaj YPS (ed) Hightech and micropropagation. Springer, Dordrecht, pp 135–149
Duhem K, Le Mercier N, Boxus P (1989) Donnés nouvelles sur l'induction et le dévelopment d'embryons somatiques chez Theobroma cacao L. Cafe Cacao Thé 33:9–14
Faria JLC, Segura J (1997) In vitro control of adventitious bud differentiation by inorganic medium components and silver thiosulfate in explants of Passiflora edulis f. flavicarpa. In Vitro Cell Dev Biol Plant 33:209–212. doi:10.1007/s11627-997-0024-8
Feder N, O’Brien TP (1968) Plant microtechnique: some principles and new methods. Am J Bot 55:123–142
Fehér A (2005) Why somatic plant cells start to form embryos? In: Mujib A, Samaj J (eds) Somatic embryogenesis, 1st edn. Springer Verlag, Berlin, pp 85–101. doi:10.1007/7089_019
Fehér A (2008) The initiation phase of somatic embryogenesis: what we know and what we don't. Acta Biol Szeged 52:53–56
Fehér A, Pasternak T, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tiss Organ Cult 74:201–228. doi:10.1023/A:1024072913021
Fernando JA, Vieira MLC, Machado SR, Appezzato-da-Gloria B (2007) New insights into the in vitro organogenesis process: the case of Passiflora. Plant Cell Tiss Organ Cult 91:37–44. doi:10.1007/s11240-007-9275-7
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158. doi:10.1016/0014-4827(68)90403-5
Garcia R, Pacheco G, Falcão E, Borges G, Mansur E (2011) Influence of type of explant, plant growth regeneration, salt composition of basal medium, and light on callogenesis and regeneration in Passiflora suberosa (Passifloraceae). Plant Cell Tiss Organ Cult 106:47–54. doi:10.1007/s11240-010-9892-4
Graham IA (2008) Seed storage oil mobilization. Ann Rev Plant Biol 59:115–142. doi:10.1146/annurev.arplant.59.032607.092938
Junqueira NTV, Braga MF, Faleiro FG, Peixoto JR, Bernacci LC (2005) Potential of wild species of passion fruit plant as resistance source to diseases. In: Faleiro FG, Junqueira NTV, Braga MF (eds) Passionfruit: germplasm and breeding, 1st edn. Embrapa Cerrados, Planaltina, pp 80–108, in Portuguese
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137–138
Kirk PW Jr (1970) Neutral red as a lipid fluorochrome. Stain Technol 45:1–4
Lombardi SP, Passos IRS, Nogueira MCS, Appezato-da-Glória B (2007) In vitro shoot regeneration from roots and leaf discs of Passiflora cincinnata Mast. Braz Arch Biol Technol 50:239–247. doi:10.1590/S1516-89132007000200009
Martin AB, Cuadrado Y, Guerra H, Gallego P, Hita O, Martin L, Dorado A, Villalobos N (2000) Differences in the contents of total sugars, starch and sucrose in embryogenic and nonembryogenic calli from Medicago arborea L. Plant Sci 154:143–151. doi:10.1016/S0168-9452(99)00251-4
Mensuali-Sodi A, Lucchesini M, Maltinti S, Serra G, Tognoni F (2007) Leaf senescence in tissue culture of Passiflora incarnata L.: the role of ethylene. In: Ramina A, Chang C, Giovannoni J, Klee H, Perata P, Woltering E (eds) Advances in plant ethylene research. Proceedings of the 7th International on the Plant Ethylene Hormone, Springer, p 151–152
Mikuła A, Tykarska T, Zielinska M, Rybczynski J, Kuras M (2004) Ultrastructural changes in zygotic embryos of Gentiana punctata (L.) during initiation and formation of callus tissue and somatic embryos. Acta Biol Cracoviensia Bot 46:1–12
Mikuła A, Tykarska T, Kuras M, Rybczynski J (2005) Somatic embryogenesis of Gentiana cruciata (L.): histological and ultrastructural changes in seedling hypocotyl explant. In Vitro Cell Dev Biol Plant 41:686–694. doi:10.1079/IVP2005678
Moura EF, Ventrella MC, Motoike SY, Sá Júnior AQ, Carvalho M, Manfio CE (2008) Histological study of somatic embryogenesis induction on zygotic embryos of macaw palm (Acrocomia aculeata (Jacq.) Lodd. ex Martius). Plant Cell Tiss Organ Cult 95:175–184. doi:10.1007/s11240-008-9430-9
Moura EF, Ventrella MC, Motoike SY (2010) Anatomy, histochemistry and ultrastructure of seed and somatic embryo of Acrocomia aculeata (Arecaceae). Sci Agric 67:399–407. doi:10.1590/S0103-90162010000400004
Müntz K (1998) Deposition of storage proteins. Plant Mol Biol 38:77–99. doi:10.1023/A:1006020208380
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Murphy DJ (2001) Biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res 40:325–438. doi:10.1016/S0163-7827(01)00013-3
O’Brien TP, McCully ME (1981) The study of plant structure principles and selected methods. Termarcarphi Pty, Melbourne
Oliveira JC, Ruggiero C (2005) Passionfruit species with agronomic potential. In: Faleiro FG, Junqueira NTV, Braga MF (eds) Passionfruit: germplasm and breeding, 1st edn. Embrapa Cerrados, Planaltina, pp 142–158, in Portuguese
Paim Pinto DL, Barros BA, Viccini LF, Campos JMS, Silva ML, Otoni WC (2010) Ploidy stability of somatic embryogenesis-derived Passiflora cincinnata Mast. plants as assessed by flow cytometry. Plant Cell Tiss Organ Cult 103:71–79. doi:10.1007/s11240-010-9756-y
Paim Pinto DL, Almeida AMR, Rêgo MM, Silva ML, Oliveira EJ, Otoni WC (2011) Somatic embryogenesis from mature zygotic embryos of commercial passionfruit (Passiflora edulis Sims) genotypes. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-011-0003-y
Pan X, Yang X, Lin G, Zou R, Chen H, Samaj J, Xu C (2011) Ultrastructural changes and the distribution of arabinogalactan proteins during somatic embryogenesis of banana (Musa spp. AAA cv. ‘Yueyoukang 1’). Physiol Plant 142:372–389. doi:10.1111/j.1399-3054.2011.01478.x
Passos IRS, Bernacci LC (2005) Tissue culture applied to in vitro germplasm conservation and breeding of passionfruit (Passiflora spp.). In: Faleiro FG, Junqueira NTV, Braga MF (eds) Passionfruit: germplasm and breeding. Embrapa Cerrados, Planaltina, pp 361–383, in Portuguese
Pearse AGE (1980) Histochemistry theoretical and applied. Churchill Livingston, Edinburgh
Perez-Núñez MT, Chan JL, Saenz L, González T, Verdeil JL, Oropeza C (2006) Improved somatic embryogenesis from Cocos nucifera (L.) plumule explants. In Vitro Cell Dev Biol Plant 42:37–43. doi:10.1079/IVP2005722
Pinto AP, Monteiro-Hara ACBA, Stipp LCL, Mendes BMJ (2010a) In vitro organogenesis of Passiflora alata. In Vitro Cell Dev Biol Plant 46:28–33. doi:10.1007/s11627-009-9251-5
Pinto G, Silva S, Araújo C, Neves L, Santos C (2010b) Histocytological changes and reserves accumulation during somatic embryogenesis in Eucalyptus globulus. Trees 24:763–769. doi:10.1007/s00468-010-0446-5
Puigderrajols P, Celestino C, Suils M, Toribio M, Molinas M (2000) Histology of organogenic and embryogenic responses in cotyledons of somatic embryos of Quercus suber L. Int J Plant Sci 161:353–362. doi:10.1086/314266
Puigderrajols P, Mir G, Molinas M (2001) Ultrastructure of early secondary embryogenesis by multicellular and unicellular pathways in cork oak (Quercus suber L.). Ann Bot 87:179–189. doi:10.1006/anbo.2000.1317
Quiroz-Figueroa FR, Fuentes-Cerda CFJ, Rojas-Herrera R, Loyola-Vargas VM (2002) Histological studies on the developmental stages and differentiation of two different somatic embryogenesis systems of Coffea arabica. Plant Cell Rep 20:1141–1149. doi:10.1007/s00299-002-0464-x
Quiroz-Figueroa FR, Rojas-Herrera R, Galaz-Avalos RM, Loyola-Vargas VM (2006) Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell Tiss Organ Cult 86:285–301. doi:10.1007/s11240-006-9139-6
Rêgo MM, Rêgo ER, Bruckner CH, Otoni WC, Pedroza CM (2010) Variation of gynogenic ability in passion fruit (Passiflora edulis Sims.) accessions. Plant Breed 130:86–91. doi:10.1111/j.1439-0523.2009.01746.x
Rêgo MM, Rêgo ER, Bruckner CH, Finger FL, Otoni WC (2011) In vitro induction of autotetraploids from diploid yellow passion fruit mediated by colchicine and oryzalin. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-011-9995-6
Reis LB, Silva ML, Lima ABP, Oliveira MLP, Paim Pinto DL, Lani ERG, Otoni WC (2007) Agrobacterium rhizogenes-mediated transformation of passionfruit species: Passiflora cincinnata and P. edulis flavicarpa. Acta Hort 738:425–431
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208
Rodriguez APM, Wetzstein HY (1998) A morphological and histological comparison of the initiation and development of pecan (Carya illionensis) somatic embryogenesis cultures induced with naphthaleneacetic acid or 2,4- dichlorophenoxyacetic acid. Protoplasma 204:71–83. doi:10.1007/BF01282295
Rose RJ, Mantiri FR, Kurdyukov S, Chen S-K, Wang X-D, Nolan KE, Sheahan MB (2010) Developmental biology of somatic embryogenesis. In: Pua E-C, Davey MR (eds) Plant developmental biology-biotechnological perspectives, vol 2. Springer, Heidelberg, pp 3–26. doi:10.1007/978-3-642-04670-4_1
Sané D, Aberlenc-Bertoss F, Gassama-Dia YK, Sagna M, Trouslot MF, Duval Y, Borgel A (2006) Histocytological analysis of callogenesis and somatic embryogenesis from cell suspensions of date palm (Phoenix dactylifera). Ann Bot 98:301–308. doi:10.1093/aob/mcl104
San-José MC, Corredoira E, Martínez MT, Vidal N, Valladares S, Mallón R, Vieitez AM (2010) Shoot apex explants for induction of somatic embryogenesis in mature Quercus robur L. trees. Plant Cell Rep 29:661–671. doi:10.1007/s00299-010-0852-6
Shang HH, Liu CL, Zhang CJ, Li FL, Hong WD, Li FG (2009) Histological and ultrastructural observation reveals significant cellular differences between Agrobacterium transformed embryogenic and non-embryogenic calli of cotton. J Integr Plant Biol 51:456–465. doi:10.1111/j.1744-7909.2009.00824.x
Silva ML, Paim Pinto DL, Guerra MP, Floh EIS, Bruckner CH, Otoni WC (2009) A novel regeneration system for wild passion fruit species (Passiflora cincinnata Mast.) based on somatic embryogenesis from mature zygotic embryos. Plant Cell Tiss Organ Cult 99:47–54. doi:10.1007/s11240-009-9574-2
Silva CV, Oliveira LS, Loriato VAP, Silva LC, Campos JMS, Viccini LF, Oliveira EJ, Otoni WC (2011) Organogenesis from root explants of commercial populations of Passiflora edulis Sims and a wild passionfruit species, P. cincinnata Masters. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-011-9991-x
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43. doi:10.1016/S0022-5320(69)90033-1
Trevisan F, Mendes BMJ, Maciel SC, Vieira MLC, Meleti LMM, Rezende JAM (2006) Resistance to Passion fruit woodiness virus in transgenic passionflower expressing the virus coat protein gene. Plant Dis 90:1026–1030. doi:10.1094/PD-90-1026
Verdeil JL, Hocher V, Huet C, Grosdemange F, Escoute J, Michaux-Ferriere N, Nicole M (2001) Ultrastructural changes in coconut calli associated with the acquisition of embryogenic competence. Ann Bot 88:9–18. doi:10.1006/anbo.2001.1408
Vidal BC (1977) Acid glycosaminoglycans and endochondral ossification microespectrophotometric evaluation and macromolecular orientation. Cell Mol Biol 22:45–64
Vieira MLC, Carneiro MS (2004) Passiflora spp., passionfruit. In: Litz RE (ed) Biotechnology of fruit and nut crops, 1st edn. CABI, Oxford, pp 435–453
Zerbini FM, Otoni WC, Vieira MLC (2008) Passionfruit. In: Kole C, Hall TC (eds) A compendium of transgenic crop plants, v.5, Tropical and subtropical fruit and nuts, 1st edn. Wiley, Berlin, pp 213–234
Zienkiewicz A, Jiménez-López JC, Zienkiewicz K, Alché JD, Rodríguez-García MI (2011) Development of the cotyledon cells during olive (Olea europaea L.) in vitro seed germination and seedling growth. Protoplasma. doi:10.1007/s00709-010-0242-5
Acknowledgments
The authors would like to thank CAPES, CNPq, and FAPEMIG for the financial support, Research Support Center/Electron Microscopy Applied to Agriculture (NAP/MEPA-ESALQ/USP), and Dr. Andréa Dias Koehler and the anonymous reviewers for their criticisms and valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Alexander Schulz
Rights and permissions
About this article
Cite this article
Rocha, D.I., Vieira, L.M., Tanaka, F.A.O. et al. Somatic embryogenesis of a wild passion fruit species Passiflora cincinnata Masters: histocytological and histochemical evidences. Protoplasma 249, 747–758 (2012). https://doi.org/10.1007/s00709-011-0318-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-011-0318-x