Abstract
Objectives
Optimal selection of idiopathic normal pressure hydrocephalus (iNPH) patients for shunt surgery is challenging. Disease State Index (DSI) is a statistical method that merges multimodal data to assist clinical decision-making. It has previously been shown to be useful in predicting progression in mild cognitive impairment and differentiating Alzheimer’s disease (AD) and frontotemporal dementia. In this study, we use the DSI method to predict shunt surgery response for patients with iNPH.
Methods
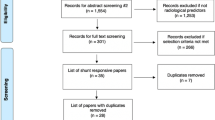
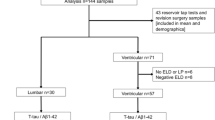
In this retrospective cohort study, a total of 284 patients (230 shunt responders and 54 non-responders) from the Kuopio NPH registry were analyzed with the DSI. Analysis included data from patients’ memory disorder assessments, age, clinical symptoms, comorbidities, medications, frontal cortical biopsy, CT/MRI imaging (visual scoring of disproportion between Sylvian and suprasylvian subarachnoid spaces, atrophy of medial temporal lobe, superior medial subarachnoid spaces), APOE genotyping, CSF AD biomarkers, and intracranial pressure.
Results
Our analysis showed that shunt responders cannot be differentiated from non-responders reliably even with the large dataset available (AUC = 0.58).
Conclusions
Prediction of the treatment response in iNPH is challenging even with our extensive dataset and refined analysis. Further research of biomarkers and indicators predicting shunt responsiveness is still needed.


Similar content being viewed by others
Abbreviations
- ACC:
-
Accuracy
- AD:
-
Alzheimer’s disease
- AUC:
-
Area under the receiver-operator curve
- Aβ:
-
Amyloid-beta
- Aβ42:
-
Amyloid-beta 42
- BMI:
-
Body mass index
- CDR:
-
Clinical dementia rating
- CT:
-
Computed tomography
- CSF:
-
Cerebrospinal fluid
- DSI:
-
Disease state index
- HPτ:
-
Hyperphosphorylated tau
- ICP:
-
Intracranial pressure
- iNPH:
-
Idiopathic normal pressure hydrocephalus
- KUH:
-
Kuopio University Hospital
- MMSE:
-
Mini-Mental State Examination
- NPH:
-
Normal pressure hydrocephalus
- VPS:
-
Ventriculoperitoneal shunt
References
Alafuzoff I, Pikkarainen M, Arzberger T, Thal DR, Al-Sarraj S, Bell J, Bodi I, Budka H, Capetillo-Zarate E, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Kavantzas N, King A, Korkolopoulou P, Kovács GG, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Stadelmann C, Streichenberger N, Tagliavini F, Kretzschmar H (2008) Inter-laboratory comparison of neuropathological assessments of β-amyloid protein: a study of the BrainNet Europe consortium. Acta Neuropathol 115(5):533–546
Brean A, Eide PK (2008) Assessment of idiopathic normal pressure patients in neurological practice: the role of lumbar infusion testing for referral of patients to neurosurgery. Eur J Neurol 15(6):605–612
Delwel EJ, de Jong DA, Avezaat CJJ (2005) The prognostic value of clinical characteristics and parameters of cerebrospinal fluid hydrodynamics in shunting for idiopathic normal pressure hydrocephalus. Acta Neurochir (Wien) 147(10):1037–1043
Eide PK, Brean A (2006) Intracranial pulse pressure amplitude levels determined during preoperative assessment of subjects with possible idiopathic normal pressure hydrocephalus. Acta Neurochir (Wien) 148(11):1151–1156
Eide P, Pripp A (2014) Increased prevalence of cardiovascular disease in idiopathic normal pressure hydrocephalus patients compared to a population-based cohort from the HUNT3 survey. Fluids Barriers CNS 11(1):19
Eide PK, Sorteberg W (2010) Diagnostic intracranial pressure monitoring and surgical management in idiopathic normal pressure hydrocephalus. Neurosurgery 66(1):80–91
Elobeid A, Laurell K, Cesarini KG, Alafuzoff I (2015) Correlations between mini-mental state examination score, cerebrospinal fluid biomarkers, and pathology observed in brain biopsies of patients with normal-pressure hydrocephalus. J Neuropathol Exp Neurol 74(5):470–479
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198
Foss T, Eide PK, Finset A (2007) Intracranial pressure parameters in idiopathic normal pressure hydrocephalus patients with or without improvement of cognitive function after shunt treatment. Dement Geriatr Cogn Disord 23(1):47–54
Gölz L, Ruppert F-H, Meier U, Lemcke J (2014) Outcome of modern shunt therapy in patients with idiopathic normal pressure hydrocephalus 6 years postoperatively. J Neurosurg 121(4):771–775
Hall A, Mattila J, Koikkalainen J, Lotjonen J, Wolz R, Scheltens P, Frisoni G, Tsolaki M, Nobili F, Freund-Levi Y, Minthon L, Frolich L, Hampel H, Visser P, Soininen H (2015) Predicting progression from cognitive impairment to Alzheimer’s disease with the Disease State Index. Curr Alzheimer Res 12(1):69–79
Hall A, Muñoz-Ruiz M, Mattila J, Koikkalainen J, Tsolaki M, Mecocci P, Kloszewska I, Vellas B, Lovestone S, Visser PJ, Lötjonen J, Soininen H, Alzheimer Disease Neuroimaging Initiative, AddNeuroMed consortium, DESCRIPA and Kuopio L-MCI (2015) Generalizability of the disease state index prediction model for identifying patients progressing from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis 44(1):79–92
Hamilton R, Patel S, Lee EB, Jackson EM, Lopinto J, Arnold SE, Clark CM, Basil A, Shaw LM, Xie SX, Grady MS, Trojanowski JQ (2010) Lack of shunt response in suspected idiopathic normal pressure hydrocephalus with Alzheimer disease pathology. Ann Neurol 68(4):535–540
Hashimoto M, Ishikawa M, Mori E, Kuwana N (2010) Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: a prospective cohort study. Cerebrospinal Fluid Res 7(1):18
Hulstaert F, Blennow K, Ivanoiu A, Schoonderwaldt HC, Riemenschneider M, Deyn PPD, Bancher C, Cras P, Wiltfang J, Mehta PD, Iqbal K, Pottel H, Vanmechelen E, Vanderstichele H (1999) Improved discrimination of AD patients using -amyloid(1–42) and tau levels in CSF. Neurology 52(8):1555–1555
Ishikawa M, Hashimoto M, Mori E, Kuwana N, Kazui H (2012) The value of the cerebrospinal fluid tap test for predicting shunt effectiveness in idiopathic normal pressure hydrocephalus. Fluids Barriers CNS 9(1):1
Junkkari A, Sintonen H, Nerg O, Koivisto AM, Roine RP, Viinamäki H, Soininen H, Jääskeläinen JE, Leinonen V (2015) Health-related quality of life in patients with idiopathic normal pressure hydrocephalus. Eur J Neurol 22(10):1391–1399
Kazui H, Mori E, Ohkawa S, Okada T, Kondo T, Sakakibara R, Ueki O, Nishio Y, Ishii K, Kawaguchi T, Ishikawa M, Takeda M (2013) Predictors of the disappearance of triad symptoms in patients with idiopathic normal pressure hydrocephalus after shunt surgery. J Neurol Sci 328(1–2):64–69
Kitagaki H, Mori E, Ishii K, Yamaji S, Hirono N, Imamura T (1998) CSF spaces in idiopathic normal pressure hydrocephalus: morphology and volumetry. AJNR Am J Neuroradiol 19(7):1277–1284
Klinge P, Marmarou A, Bergsneider M, Relkin N, Black PM (2005) Outcome of shunting in idiopathic normal-pressure hydrocephalus and the value of outcome assessment in shunted patients. Neurosurgery 57(3):S2–40–S2–52
Koivisto AM, Alafuzoff I, Savolainen S, Sutela A, Rummukainen J, Kurki M, Jääskeläinen JE, Soininen H, Rinne J, Leinonen V (2013) Poor cognitive outcome in shunt-responsive idiopathic normal pressure hydrocephalus. Neurosurgery 72(1):1–8
Kojoukhova M, Koivisto AM, Korhonen R, Remes AM, Vanninen R, Soininen H, Jääskeläinen JE, Sutela A, Leinonen V (2015) Feasibility of radiological markers in idiopathic normal pressure hydrocephalus. Acta Neurochir (Wien) 157(10):1709–1719
Leinonen V, Koivisto AM, Savolainen S, Rummukainen J, Sutela A, Vanninen R, Jääskeläinen JE, Soininen H, Alafuzoff I (2012) Post-mortem findings in 10 patients with presumed normal-pressure hydrocephalus and review of the literature. Neuropathol Appl Neurobiol 38(1):72–86
Malm J, Graff-Radford NR, Ishikawa M, Kristensen B, Leinonen V, Mori E, Owler BK, Tullberg M, Williams M a, Relkin NR (2013) Influence of comorbidities in idiopathic normal pressure hydrocephalus—research and clinical care. A report of the ISHCSF task force on comorbidities in INPH. Fluids Barriers CNS 10(1):22
Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM (2005) The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 57(3 Suppl):S2–17–S2–28
Mattila J, Koikkalainen J, Virkki A, Simonsen A, van Gils M, Waldemar G, Soininen H, Lötjönen J, Alzheimer’s Disease Neuroimaging Initiative (2011) A disease state fingerprint for evaluation of Alzheimer’s disease. J Alzheimers Dis 27(1):163–176
Mattila J, Soininen H, Koikkalainen J, Rueckert D, Wolz R, Waldemar G, Lötjönen J (2012) Optimizing the diagnosis of early Alzheimer’s disease in mild cognitive impairment subjects. J Alzheimers Dis 32(4):969–979
McGirt MJ, Woodworth G, Coon AL, Thomas G, Williams MA, Rigamonti D (2005) Diagnosis, treatment, and analysis of long-term outcomes in idiopathic normal-pressure hydrocephalus. Neurosurgery 57(4):699–705
Morris JC (1993) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43(11):2412–2412
Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C (1989) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39(9):1159–1165
Muñoz-Ruiz MÁ, Hartikainen P, Hall A, Mattila J, Koikkalainen J, Herukka S-K, Julkunen V, Vanninen R, Liu Y, Lötjönen J, Soininen H (2013) Disease state fingerprint in frontotemporal degeneration with reference to Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis 35(4):727–739
Pyykko OT, Helisalmi S, Koivisto AM, Molsa JAA, Rummukainen J, Nerg O, Alafuzoff I, Savolainen S, Soininen H, Jaaskelainen JE, Rinne J, Leinonen V, Hiltunen M (2012) APOE4 predicts amyloid- in cortical brain biopsy but not idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 83(11):1119–1124
Pyykkö OT, Lumela M, Rummukainen J, Nerg O, Seppälä TT, Herukka S-K, Koivisto AM, Alafuzoff I, Puli L, Savolainen S, Soininen H, Jääskeläinen JE, Hiltunen M, Zetterberg H, Leinonen V (2014) Cerebrospinal fluid biomarker and brain biopsy findings in idiopathic normal pressure hydrocephalus. PLoS One 9(3):e91974
Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM (2005) Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 57(3):S2–4–S2–16
Savolainen S, Hurskainen H, Paljärvi L, Alafuzoff I, Vapalahti M (2002) Five-year outcome of normal pressure hydrocephalus with or without a shunt: predictive value of the clinical signs, neuropsychological evaluation and infusion test. Acta Neurochir (Wien) 144(6):515–523
Seppala TT, Nerg O, Koivisto AM, Rummukainen J, Puli L, Zetterberg H, Pyykko OT, Helisalmi S, Alafuzoff I, Hiltunen M, Jaaskelainen JE, Rinne J, Soininen H, Leinonen V, Herukka SK (2012) CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology 78(20):1568–1575
Tarnaris A, Kitchen ND, Watkins LD (2009) Noninvasive biomarkers in normal pressure hydrocephalus: evidence for the role of neuroimaging. J Neurosurg 110(5):837–851
Thomas G, McGirt MJ, Woodworth G, Heidler J, Rigamonti D, Hillis AE, Williams MA (2005) Baseline neuropsychological profile and cognitive response to cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord 20(2–3):163–168
Tisell M, Tullberg M, Hellström P, Edsbagge M, Högfeldt M, Wikkelsö C (2011) Shunt surgery in patients with hydrocephalus and white matter changes. J Neurosurg 114(5):1–7
Virhammar J, Laurell K, Cesarini KG, Larsson E-M (2014) Preoperative prognostic value of MRI findings in 108 patients with idiopathic normal pressure hydrocephalus. Am J Neuroradiol 35(12):2311–2318
Acknowledgments
We would like to thank Marita Voutilainen, RN, for maintaining the NPH registry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Academy of Finland (decision no 263193), VTR grant V16001 of Kuopio University Hospital, The Finnish Medical Foundation, Sigrid Juselius Foundation, Maire Taponen Foundation, the Strategic Funding of the University of Eastern Finland (UEF-Brain), VPH-DARE@IT project funded by European Union’s Seventh Framework Programme (FP7/2007-2013) grant agreement no. 601055, From Patient Data to Clinical Diagnosis in Neurodegenerative Diseases PredictND project funded by the European Union’s Seventh Framework Programme (FP7/2007-2013) grant agreement no. 611005, and is part of the BIOMARKAPD project in the frame of JPND. The sponsors had no role in the design or conduct of this research.
Conflict of interest
J. Mattila and J. Lötjönen report that VTT Technical Research Centre of Finland owns the patents (U.S. Patent No. 7,840,510, Inventors: JL; PCT/FI2010/050545, pending, Inventors: JM, JL) that cover parts of the methods presented in the paper.
All other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Brain biopsy and invasive ICP measurement were part of clinical routine. The Finnish National Supervisory Authority for Welfare and Health has approved to use that information for the research purposes in cases the informed consent was not available. Informed consents were obtained from all patients for APOE genotyping and AD biomarker assessments.
Additional information
Comments
This Finnish group has developed a statistical method, the Disease State Index (DSI), which merges multimodal data to assist in clinical decision-making. The authors have so far published nine articles on the method since 2011. From these publications, it seems as if this tool has the power to predict progression, etc., in patients with Alzheimer’s disease. In the present study, the DSI method was used in an attempt to predict the response to shunt surgery in iNPH patients—and the authors found that it does not. Predicting outcome after shunt surgery in these patients is still a challenge. Although negative, these authors’ attempt at introducing a new tool was based on a fair assumption and therefore deserves to be published.
Knut Wester
Bergen, Norway
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 231 kb)
Rights and permissions
About this article
Cite this article
Luikku, A.J., Hall, A., Nerg, O. et al. Multimodal analysis to predict shunt surgery outcome of 284 patients with suspected idiopathic normal pressure hydrocephalus. Acta Neurochir 158, 2311–2319 (2016). https://doi.org/10.1007/s00701-016-2980-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-2980-4