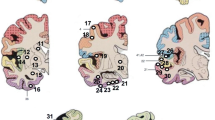
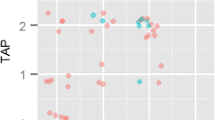
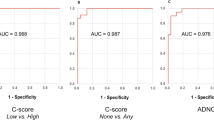
Abstract
Amyloid-β-protein (Aβ) is generally assessed by neuropathologists in diagnostics. This BrainNet Europe (http://www.brainnet-europe.org/) (15 centres and 26 participants) study was carried out to investigate the reliability of such an assessment. In the first part of this trial, tissue microarray sections were stained with the antibody of each centre’s choice. Reflecting the reality, seven antibodies and a plethora of pretreatment strategies were used. Ninety-two percent of the stainings were of good/acceptable quality and the estimation of presence of Aβ aggregates yielded good results. However, a poor agreement was reached particularly regarding quantitative (density) and qualitative (diffuse/cored plaques) results. During a joint meeting, the clone 4G8 was determined to label best the fleecy/diffuse plaques, and thus, this clone and the formic acid pretreatment technique were selected for the second part of this study. Subsequently, all stained sections were of good/acceptable quality and again a high level of concordance of the dichotomized (presence/absence) assessment of plaques and CAA was achieved. However, even when only one antibody was used, the type of Aβ-aggregates (diffuse/cored), type of vessel and Vonsattel grade, were not reliably assigned. Furthermore, the quantification of lesions was far from reliable. In line with the first trial, the agreement while assessing density (some, moderate and many) was unimpressive. In conclusion, we can confirm the utility of immunohistochemical detection of Aβ-protein in diagnostics and research. It is noteworthy that to reach reproducible results a dichotomized assessment of Aβ-immunoreactivity rather than quantification and assignment of various types of lesions should be applied, particularly when comparing results obtained by different neuropathologists.







Similar content being viewed by others
References
Akiyama H, Mori H, Saido T, Kondo H, Ikeda K, McGeer PL (1999) Occurrence of the diffuse amyloid beta-protein (Abeta) deposits with numerous Abeta-containing glial cells in the cerebral cortex of patients with Alzheimer’s disease. Glia 15:324–331
Alafuzoff I, Helisalmi S, Mannermaa A, Riekkinen P Sr, Soininen H (1999) Beta-amyloid load is not influenced by the severity of cardiovascular disease in aged and demented patients. Stroke 30:613–618
Alafuzoff I, Pikkarainen M, Al-Sarraj S, Arzberger T, Bell J, Bodi I, Bogdanovic N, Budka H, Bugiani O, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Hauw JJ, Kamphorst W, King A, Kopp N, Korkolopoulou P, Kovacs GG, Meyronet D, Parchi P, Patsouris E, Preusser M, Ravid R, Roggendorf W, Seilhean D, Streichenberger N, Thal DR, Kretzschmar H (2006) Interlaboratory comparison of assessments of Alzheimer disease-related lesions: a study of the BrainNet Europe Consortium. J Neuropathol Exp Neurol 65:740–757
Alafuzoff I, Parkkinen L, Al-Sarraj S, Arzberger T, Bell J, Bodi I, Bogdanovic I, Budka H, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Kamphorst W, King A, Korkolopoulou P, Kovacs GG, Larionov S, Meyronet D, Monoranu C, Morris J, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Streichenberger N, Thal DR, Kretzschmar H (2008) Assessment of immunohistochemically detectable α-synuclein pathology. J Neuropathol Exp Neurol 67:125–143
Allsop D, Christie G, Gray C, Holmes S, Markwell R, Owen D, Smith L, Wadsworth H, Ward RV, Hartmann T, Lichtenthaler SF, Evin G, Fuller S, Masters CL, Beyreuther K, Roberts GW (1997) Studies on inhibition of β-amyloid formation in APP751-transfected IMR-32 cells and SPA4CT-transfected SHSY5Y cells. In: Iqbal K, Winblad B, Nishimura T, Takeda M, Wisniewski HM (eds) Alzheimer’s disease: biology, diagnostics and therapeutics. Wiley, New York, pp 717–727
Arriagada PV, Marzloff K, Hyman BT (1992) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 42:1681–1688
Bennhold H (1922) Eine specifische Amyloidfärbung mit Kongorot. Münch Med Wochenschr 44:1537–1538
Burns J, Pennock CA, Stoward PJ (1967) The specificity of the staining of amyloid deposits with Thioflavine T. J Pathol Bacteriol 94:337–344
Croisier E, MRes DE, Deprez M, Goldring K, Dexter DT, Pearce RK, Graeber MB, Roncaroli F (2006) Comparative study of commercially available anti-alpha-synuclein antibodies. Neuropathol Appl Neurobiol 32:351–356
Glenner GG, Wong CW (1984) Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 16:885–890
Gouras GK, Almeida CG, Takahashi RH (2005) Intraneuronal Abeta accumulation and origin of plaques in Alzheimer’s disease. Neurobiol Aging 26:1235–1244
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 19:353–356
Hucker GJ (1921) A new modification and application of the Gram stain. J Bacteriol 6:395–397
Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y (1994) Visualization of Abeta 42(43) and Abeta 40 in senile plaques with end-specific Abeta monoclonals: evidence that an initially deposited species is Abeta 42(43). Neuron 13:45–53
Iwatsubo T, Saido TC, Mann DM, Lee VM, Trojanowski JQ (1996) Full-length amyloid-beta (1–42(43)) and amino-terminally modified and truncated amyloid-beta 42(43) deposit in diffuse plaques. Am J Pathol 149:1823–1830
Kallioniemi OP, Wagner U, Kononen J, Sauter G (2001) Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet 10:657–662
Kauppinen T, Martikainen P, Alafuzoff I (2006) Human postmortem brain tissue and 2-mm tissue microarrays. Appl Immunohistochem Mol Morphol 14:353–359
Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 18:486–489
Kim KS, Wen GY, Bancher C, Chen CMJ, Sapienza VJ, Hong H, Wisniewski HM (1990) Detection and quantitation of amyloid B-peptide with 2 monoclonal antibodies. Neurosci Res Commun 7:113–122
Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4:844–847
LaFerla FM, Green KN, Oddo S (2007) Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci 8:499–509
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA 82:4245–4249
Matsunaga Y, Saito N, Fujii A, Yokotani J, Takakura T, Nishimura T, Esaki H, Yamada T (2002) A pH-dependent conformational transition of Abeta peptide and physicochemical properties of the conformers in the glial cell. Biochem J 1:547–556
Olichney JM, Hansen LA, Hofstetter CR, Lee JH, Katzman R, Thal LJ (2000) Association between severe cerebral amyloid angiopathy and cerebrovascular lesions in Alzheimer disease is not a spurious one attributable to apolipoprotein E4. Arch Neurol 57:869–874
Saido TC, Iwatsubo T, Mann DM, Shimada H, Ihara Y, Kawashima S (1995) Dominant and differential deposition of distinct beta-amyloid peptide species: Abeta N3(pE), in senile plaques. Neuron 14:457–466
Saido TC, Yamao-Harigaya W, Iwatsubo T, Kawashima S (1996) Amino- and carboxyl-terminal heterogeneity of beta-amyloid peptides deposited in human brain. Neurosci Lett 13:173–176
Thal DR, Ghebremedhin E, Orantes M, Wiestler OD (2003) Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol 62:1287–1301
Thal DR, Capetillo-Zarate E, Del Tredici K, Braak H (2006) The development of amyloid beta protein deposits in the aged brain. Sci Aging Knowl Environ (6):re1
Wang R, Sweeney D, Gandy SE, Sisodia SS (1996) The profile of soluble amyloid beta protein in cultured cell media: detection and quantification of amyloid beta protein and variants by immunoprecipitation–mass spectrometry. J Biol Chem 13:31894–31902
Wang H, Wang H, Zhang W, Fuller GN (2002) Tissue microarrays: applications in neuropathology research, diagnosis, and education. Brain Pathol 12:95–107
Wegiel J, Kuchna I, Nowicki K, Frackowiak J, Mazur-Kolecka B, Imaki H, Wegiel J, Mehta PD, Silverman WP, Reisberg B, Deleon M, Wisniewski T, Pirttilla T, Frey H, Lehtimäki T, Kivimäki T, Visser FE, Kamphorst W, Potempska A, Bolton D, Currie JR, Miller DL (2007) Intraneuronal Abeta immunoreactivity is not a predictor of brain amyloidosis-beta or neurofibrillary degeneration. Acta Neuropathol 113:389–402
Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP Jr (1991) Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol 30:637–649
Acknowledgments
We thank Tarja Kauppinen and all other laboratory technicians of the BNE members for their skilful technical assistance, Ewen MacDonald for critical reading of the manuscript, and Vesa Kiviniemi for his assistance in statistics. We acknowledge the following BNE centres (in alphabetical order of the cities) for contributing the material with generic data used in this study: Netherlands Brain Bank (Amsterdam), National and Capodistrian University of Athens (Athens), Hospital de Bellvitge/Universitat de Barcelona (Barcelona), Alma Mater Studiorum—Università de Bologna (Bologna), National Institute of Psychiatry and Neurology (Budapest, OPNI), The University of Edinburgh (Edinburgh), The Saarland University Hospital (Homburg), University of Kuopio (Kuopio), Imperial College of Science, Technology and Medicine (London), London Institute of Psychiatry (London), Hospices Civils de Lyon (Lyon), Istituto Nazionale Neurologico Carlo Besta (Milan), Ludwig-Maximilians-University of Munich (Munich), Medical University Vienna (Vienna) and University of Wuerzburg (Wuerzburg). This study was supported by European Union grant FP6: BNEII No LSHM-CT-2004-503039. This article reflects only authors’ views and the Community is not liable for any use that may be made of the information contained therein. The study has been authorized by the Ethics Committee of Kuopio University Hospital.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alafuzoff, I., Pikkarainen, M., Arzberger, T. et al. Inter-laboratory comparison of neuropathological assessments of β-amyloid protein: a study of the BrainNet Europe consortium. Acta Neuropathol 115, 533–546 (2008). https://doi.org/10.1007/s00401-008-0358-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0358-2