Abstract
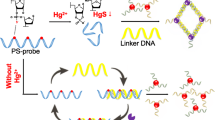
The authors describe a colorimetric method for the determination of Hg(II) ion. It is based on the color change from red to colorless as displayed by gold nanoparticle (AuNP) modified with thymine - rich DNA. Signal amplification is accomplished by free strand displacement recycling. In this strategy, Hg(II) unfolds the arch-trigger duplex due to the high affinity between Hg(II) and the thymines to form T-Hg(II)-T structures, thereby causing the release of trigger. The liberated trigger unfolds the hairpin structure of H1, and unfolded H1 further unfolds with H2. As a result, the H2 hairpin displaces trigger, and the released trigger unfolds another H1. This results in strong and enzyme-free strand displacement recycling amplification. The aggregation of DNA-AuNPs occurs in the presence of the duplex formed by hairpins H2 and H1. This results in a color change from red to colorless that can be visually observed. Under optimal conditions, the assay has a detection range over 4 orders of magnitude and a 3.4 nM detection limit. The assay is selective, sensitive, rapid and cost-effective. In our perception, it represents a useful platform for determination of Hg(II).

Schematic presentation of the simple, rapid, low cost colorimetric detection of mercury(II) based on enzyme-free strand displacement amplification along with DNA-labeled AuNP.






Similar content being viewed by others
References
Chen JH, Zhou SG, Wen JL (2014) Disposable strip biosensor for visual detection of Hg2+ based on Hg2+-triggered toehold binding and exonuclease III-assisted signal amplification. Anal Chem 86:3108–3114
Li HL, Zhai JF, Tian JQ, Luo YL, Sun XP (2011) Carbon nanoparticle for highly sensitive and selective fluorescent detection of mercury(II) ion in aqueous solution. Biosens Bioelectron 26:4656–4660
WHO (2007) Exposure to mercury: a major public health concern. WHO Preventing Disease Through Healthy Environments. Geneva, Switzerland, World Health Organization
Huang PJ, Wang F, Liu JW (2015) Cleavable molecular beacon for Hg2+ detection based on Phosphorothioate RNA modifications. Anal Chem 87:6890–6895
Office of Water (2001) Mercury update: impact on fish advisories, EPA fact sheet EPA-823-F-01−011. U.S. Environmental Protection Agency, Washington, D. C
Lo JM, Yu JC, Hutchison FI, Waei CM (1982) Solvent extraction of Dithiocarbamate complexes and back-extraction with mercury(II) for determination of trace metals in seawater by atomic absorption spectrometry. Anal Chem 54:2536–2539
Zenko Y, Masao T (1977) Indirect determination of Submicrogram amounts of sulfide by flameless atomic absorption spectrometry of mercury. Mikrochim Acta 67(5):459–468
Balint L, Vedrina-Dragojević I, Šebečić B, Momirović-Čuljat J, Horvatić M (1997) Spectrofluorometric method for determination of the Total mercury content in environmental samples-waste waters. Mikrochim Acta 127:61–65
Kim HN, Ren WX, Kim JS, Yoon JY (2012) Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem Soc Rev 41:3210–3244
Gomez-Ariza J, Lorenzo F, Garcia-Barrera T (2005) Comparative study of atomic fluorescence spectroscopy and inductively coupled plasma mass spectrometry for mercury and arsenic multispeciation. Anal Bioanal Chem 382:485–492
Gholivand MB, Parvin MH (2010) Differential pulse anodic stripping Voltammetric simultaneous determination of copper (II) and silver (I) with Bis(2-hydroxyacetophenone) butane-2,3-dihydrazone modified carbon paste electrodes. Electroanalysis 22:2291–2296
Deng W, Tan Y, Li Y, Wen Y, Su Z, Huang Z, Huang S, Meng Y, Xie Q, Luo Y, Yao S (2010) Square wave voltammetric determination of hg(II) using thiol functionalized chitosan-multiwalled carbon nanotubes nanocomposite film electrode. Microchim Acta 169:367–373
Leermakers M, Baeyens W, Quevauviller P, Horvat M (2005) Mercury in environmental samples: speciation, artifacts and validation. Trends Anal Chem 24:383–393
Ratner N, Mandler D (2015) Electrochemical detection of low concentrations of mercury in water using gold nanoparticles. Anal Chem 87:5148–5155
Xue XJ, Wang F, Liu XG (2008) One-step, room temperature, colorimetric detection of mercury (Hg2+) using DNA/nanoparticle conjugates. J Am Chem Soc 130:3244–3245
F Zarlaida, M. Adlim, (2016) Gold and silver nanoparticles and indicator dyes as active agents in colorimetric spot and strip tests for mercury(II) ions: a review; Microchim Acta 2016; on the web. DOI: 10.1007/s00604-016-1967-4
Wu S, Li Q, Duan N, Ma H, Wang Z (2016) DNA aptamer selection and aptamer-based fluorometric displacement assay for the hepatotoxin microcystin-RR. Microchim Acta 183(9):2555–2562
Zhang JF, Lim CS, Cho BR, Kim JS (2010) A two-photon excited luminescence of water-soluble rhodamine–platinum(II) complex: fluorescent probe specific for Hg2+ detection in live cell. Talanta 83:658–662
Xu Q, Du S, Jin GD, Li HB, Hu XY (2011) Determination of acetamiprid by a colorimetric method based on the aggregation of gold nanoparticles. Microchim Acta 173:323–329
Zhang J, Tang Y, Lv J, Fang SQ, Tang DP (2015) Glucometer-based quantitative determination of hg(II) using gold particle encapsulated invertase and strong thymine-hg(II)-thymine interaction for signal amplification. Microchim Acta 182:1153–1159
Shi QN, Shi YP, Pan Y, Yue ZF, Zhang H, Yi CQ (2015) Colorimetric and bare eye determination of urinary methylamphetamine based on the use of aptamers and the salt-induced aggregation of unmodified gold nanoparticles. Microchim Acta 182:505–511
Wang H, Wang YX, Jin JY, Yang RH (2008) Gold nanoparticle-based colorimetric and “turn-on” fluorescent probe for mercury(II) ions in aqueous solution. Anal Chem 80:9021–9028
Wang GF, Huang H, Zhang XJ, Wang L (2012) Electrically contacted enzyme based on dual hairpin DNA structure and its application for amplified detection of Hg2+. Biosens Bioelectron 35:108–114
Cao GX, Wu XM, Dong YM, Li ZJ, Wang GL (2016) Colorimetric determination of melamine based on the reversal of the mercury(II) induced inhibition of the light-triggered oxidase-like activity of gold nanoclusters. Microchim Acta 183:441–448
Yuan M, Zhu YG, Lou XH, Chen C, Wei G, Lan MB, Zhao JL (2012) Sensitive label-free oligonucleotide-based microfluidic detection of mercury(II)ion by using exonuclease I. Biosens Bioelectron 31:330–336
Wang GF, Xu G, Zhu YH, Zhang XJ (2014) A “turn-on” carbon nanotube–ag nanoclusters fluorescent sensor for sensitive and selective detection of Hg2+ with cyclic amplification of exonuclease III activity. Chem Commun 50:747–750
Zhu X, Zhou XM, Xing D (2011) Ultrasensitive and selective detection of mercury(II) in aqueous solution by polymerase assisted fluorescence amplification. Biosens Bioelectron 26:2666–2669
Zhu GC, Li Y, Zhang CY (2014) Simultaneous detection of mercury(II) and silver(I)ions with picomolar sensitivity. Chem Commun 50:572–574
Xu YY, Zhou WJ, Zhou M, Xiang Y, Yuan R, Chai YQ (2015) Toehold strand displacement-driven assembly of G-quadruplex DNA for enzyme-free and non-label sensitive fluorescent detection of thrombin. Biosens Bioelectron 64:306–310
Quan K, Huang J, Yang XH, Yang YJ, Ying L, Wang H, He Y, Wang KM (2015) An enzyme-free and amplified colorimetric detection strategy via target–aptamer binding triggered catalyzed hairpin assembly. Chem Commun 51:937–940
Yan FY, Kong DP, Luo YM, Ye QH, He JJ, Guo XF, Chen L (2016) Carbon dots serve as an effective probe for the quantitative determination and for intracellular imaging of mercury(II). Microchim Acta 183:1611–1618
Lu Y, Yu J, Ye WC, Yao X, Zhou PP, Zhang HX, Zhao SQ, Jia LP (2016) Spectrophotometric determination of mercury(II) ions based on their stimulation effect on the peroxidase-like activity of molybdenum disulfide nanosheets. Microchim Acta 183:2481–2489
Shi DC, Yan FY, Zhou XG, Zheng TC, Shi YY, Fu WG, Chen L (2016) Preconcentration and fluorometric detection of mercury ions using magnetic core-shell chitosan microspheres modified with a rhodamine spirolactam. Microchim Acta 183:319–327
Mojtaba S, Afsaneh S, Zahra M, Raheleh A (2016) Highly selective aggregation assay for visual detection of mercury ion based on competitive binding of sulfur-doped carbon nanodots to gold nanoparticles and mercury ions. Microchim Acta 183:2327–2335
Khosro ZK, Alagarsamy P, Subramaniam J, Ramasamy R, Hong NL, Boon HO, SDB C, Yeh YK, Huang NM (2016) Amalgamation based optical and colorimetric sensing of mercury(II) ions with silver@graphene oxide nanocomposite materials. Microchim Acta 183:369–377
Hu TY, Yan X, Na WD, Su XG (2016) Aptamer-based aggregation assay for mercury(II) using gold nanoparticles and fluorescent CdTe quantum dots. Microchim Acta 183:2131–2137
Tang WJ, Wang Y, Wang PP, Di JW, Yang JP, Wu Y (2016) Synthesis of strongly fluorescent carbon quantum dots modified with polyamidoamine and a triethoxysilane as quenchable fluorescent probes for mercury(II). Microchim Acta 183:2571–2578
An’amt MN, Perumal R, Huang NM, Wei LS (2016) Visual and spectrophotometric determination of mercury(II) using silver nanoparticles modified with graphene oxide. Microchim Acta 183:597–603
Acknowledgements
This work was supported by NSFC (21405060, 1471644), Shandong Province Natural Science Funds for Distinguished Young Scholars (JQ201410), and Shandong Province Natural Science Funds (ZR2015CM027).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 680 kb)
Rights and permissions
About this article
Cite this article
Liu, S., Leng, X., Wang, X. et al. Enzyme-free colorimetric assay for mercury(II) using DNA conjugated to gold nanoparticles and strand displacement amplification. Microchim Acta 184, 1969–1976 (2017). https://doi.org/10.1007/s00604-017-2182-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2182-7