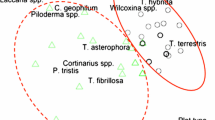
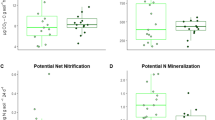
Abstract
After stand-replacing disturbance, regenerating conifer seedlings become colonized by different ectomycorrhizal fungi (EMF) than the locally adapted EMF communities present on seedlings in mature forests. We studied whether EMF species that colonized subalpine fir (Abies lasiocarpa) seedlings in clearcuts differed from those that colonized seedlings in adjacent mature forests with respect to mycorrhizoplane extracellular enzyme activities (EEAs) and N status of the seedlings. We tested two alternate hypotheses: (1) that EEAs would differ between the two EMF communities, with higher activities associated with forest-origin communities, and (2) that acclimation to soil environment was considerable enough that EEAs would be determined primarily by the soil type in which the ectomycorrhizas were growing. Naturally colonized fir seedlings were reciprocally transplanted between clearcuts and forests, carrying different EMF communities with them. EEAs were influenced more by destination environment than by EMF community. EEAs were as high in early-successional as in late-successional communities in both destination environments. Buds of clearcut-origin seedlings had the same or higher N contents as forest seedlings after a growing season in either environment. These results indicate that (i) symbiotic EMF and/or their associated microbial communities demonstrate substantial ability to acclimate to new field environments; (ii) the ability to produce organic matter-degrading enzymes is not a trait that necessarily distinguishes early- and late-successional EMF communities in symbiosis; (iii) early-successional EMF are as capable of supporting seedling N accumulation in forest soils as late-successional EMF; and (iv) disturbed ecosystems where early-successional EMF are present should have high resilience for organic matter degradation.







Similar content being viewed by others
References
Agerer R (1987-2002) Colour atlas of ectomycorrhizae. Einhorn-Verlag Eduard Dietenberger, Schwäbisch Gmünd
Barker JS, Simard SW, Jones MD, Durall DM (2013) Ectomycorrhizal fungal community assembly on regenerating Douglas-fir after wildfire and clearcut harvesting. Oecologia 172:1179–1189
Blaalid R, Davey ML, Kauserud H, Carlsen T, Halvorsen R, Hoiland K, Eidesen PB (2014) Arctic root-associated fungal community composition reflects environmental filtering. Mol Ecol 23:649–659
Bödeker ITM, Nygren CMR, Taylor AFS, Olson A, Lindahl BD (2009) Class II peroxidase-encoding genes are present in a phylogenetically wide range of ectomycorrhizal fungi. ISME J 3:1387–1395
Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R. Springer, New York
Brzostek ER, Finzi AC (2011) Substrate supply, fine roots, and temperature control proteolytic enzyme activity in temperate forest soils. Ecology 92:892–902
Buée M, Courty PE, Mignot D, Garbaye J (2007) Soil niche effect on species diversity and catabolic activities in an ectomycorrhizal fungal community. Soil Biol Biochem 39:1947–1955
Burke DJ, Smemo KA, Lopez-Gutierrez JC, Hewins CR (2012) Soil enzyme activity in an old-growth northern hardwood forest: interactions between soil environment, ectomycorrhizal fungi and plant distribution. Pedobiologia 55:357–364
Burke DJ, Weintraub MN, Hewins CR, Kalisz S (2011) Relationship between soil enzyme activities, nutrient cycling and soil fungal communities in a northern hardwood forest. Soil Biol Biochem 43:795–803
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H, Stenlid J, Finlay RD, Wardle DA, Lindahl BD (2013) Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339:1615–1618
Colpaert JV, van Laere A, van Assche JA (1996) Carbon and nitrogen allocation in ectomycorrhizal and non-mycorrhizal Pinus sylvestris L seedlings. Tree Physiol 16:787–793
Conn C, Dighton J (2000) Litter quality influences on decomposition, ectomycorrhizal community structure and mycorrhizal root surface acid phosphatase activity. Soil Biol Biochem 32:489–469
Coupé R, Stewart AC, Wikeem BM (1991) Engelmann spruce–subalpine fir zone. In: Meidinger D, Pojar J (eds) Ecosystems of British Columbia. Research branch. B.C. Ministry of Forests, Victoria, pp. 223–236
Courty P-E, Munoz F, Selosse M-A, Duchemin M, Criquet S, Ziarelli F, Buée M, Plassard C, Taudière A, Garbaye J, Richard F (2016) Into the functional ecology of ectomycorrhizal communities: environmental filtering of enzymatic activities. J Ecol doi. doi:10.1111/1365-2745.12633
Courty P-E, Pritsch K, Schloter M, Hartmann A, Garbaye J (2005) Activity profiling of ectomycorrhiza communities in two forest soils using multiple enzymatic tests. New Phytol 167:309–319
Deacon JW, Fleming LV (1992) Interactions of ectomycorrhizal fungi. In: Allen MF (ed) Mycorrhizal functioning: an integrative plant-fungal process. Chapman and Hall, New York, pp. 249–300
Finlay RD, Frostegård A, Sonnerfeldt AM (1992) Utilization of organic and inorganic nitrogen sources of ectomycorrhizal fungi in pure culture and in symbiosis with Pinus contorta Dougl. ex Loud. New Phytol 120:105–115
Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martínez AT, Otillar R, Spatafora JW, Yadavet JS et al (2012) The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336:1715–1719
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Godbold DL, Hoosbeek MR, Lukac M, Cotrufo MF, Janssens IA, Ceulemans R, Polle A, Velthorst EJ, Scarascia-Mugnozza G, De Angelis P, Miglietta F, Peressotti A (2006) Mycorrhizal hyphal turnover as a dominant process for carbon input into soil organic matter. Plant Soil 281:15–24
Goodman DM, Durall DM, Trofymow JA, Berch SM (1996) A manual of concise descriptions of North American ectomycorrhizae. Mycologue Publications, Victoria
Hagerman SM, Jones MD, Bradfield GE, Gillespie M, Durall DM (1999) Effects of clear-cut logging on the diversity and persistence of ectomycorrhizae at a subalpine forest. Can J For Res 29:124–134
Hannam KD, Prescott CE (2003) Soluble organic nitrogen in forests and adjacent clearcuts in British Columbia, Canada. Can J For Res 33:1709–1718
Hope GD (2009) Clearcut harvesting effects on soil and creek inorganic nitrogen in high elevation forests of southern interior British Columbia. Can J Soil Sci 89:35–44
Jarvis SG, Woodward S, Taylor AFS (2015) Strong altitudinal partitioning in the distributions of ectomycorrhizal fungi along a short (300 m) elevation. New Phytol 206:1145–1155
Jones MD, Durall DM, Cairney JWG (2003) Ectomycorrhizal fungal communities in young forest stands regenerating after clearcut logging. New Phytol 157:399–422
Jones MD, Durall DM, Harniman SMK, Classen DC, Simard SW (1997) Ectomycorrhizal diversity of Betula papyrifera and Pseudotsuga menziesii seedlings grown in the greenhouse or in single-species and mixed plots in southern British Columbia. Can J For Res 27:1872–1889
Jones MD, Grenon F, Peat H, Fitzgerald M, Holt L, Philip LJ, Bradley R (2009) Differences in 15N uptake amongst spruce seedlings colonized by three pioneer ectomycorrhizal fungi in the field. Fungal Ecol 2:110–120
Jones MD, Phillips LA, Treu R, Ward V, Berch SM (2012) Functional responses of ectomycorrhizal fungal communities to long-term fertilization of lodgepole pine (Pinus contorta Dougl. ex Loud. var. latifolia Engelm.) stands in Central British Columbia. Appl Soil Ecol 60:29–40
Jones MD, Twieg BD, Ward V, Barker J, Durall DM, Simard SW (2010) Functional complementarity of Douglas-fir ectomycorrhizas for extracellular enzyme activity after wildfire or clearcut logging. Funct Ecol 24:1139–1151
Jumpponen A, Jones KL (2009) Massively parallel 454-sequencing indicates hyperdiverse fungal communities in temperate Quercus macrocarpa phyllosphere. New Phytol 184:438–448
Kataja-aho S, Pennanen T, Lensu A, Haimi J (2012) Does stump removal affect early growth and mycorrhizal infection of spruce (Picea abies) seedlings in clear-cuts? Scand J Forest Res 27:746–753
Keller G (1996) Utilization of inorganic and organic nitrogen sources by high-subalpine ectomycorrhizal fungi of Pinus cembra in pure culture. Mycol Res 100:989–998
Kennedy PG, Bruns TD (2005) Priority effects determine the outcome of ectomycorrhizal competition between two Rhizopogon species colonizing Pinus muricata seedlings. New Phytol 166:631–638
Klein T, Siegwolf RTW, Körner C (2016) Belowground carbon trade among tall trees in a temperate forest. Science 352:342–344
Kohler A, Kuo A, Nagy LG, Morin E, Barry KW, Buscot F, Canbäck B, Choi C, Cichocki N, Clum A et al (2015) Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat Genet 47:410–415
Kotroczó Z, Veres Z, Fekete I, Krakomperger Z, Tóth JA, Lajtha K, Tóthmérész B (2014) Soil enzyme activity in response to long-term organic matter manipulation. Soil Biol Biochem 70:237–243
Kranabetter JM (2004) Ectomycorrhizal community effects on hybrid spruce seedling growth and nutrition in clearcuts. Can J Bot 82:983–991
Kranabetter JM, Durall DM, MacKenzie WH (2009) Diversity and species distribution of ectomycorrhizal fungi along productivity gradients of a southern boreal forest. Mycorrhiza 19:99–111
Kranabetter JM, Friesen J (2002) Ectomycorrhizal community structure on western hemlock (Tsuga heterophylla) seedlings transplanted from forests into openings. Can J Bot 80:861–868
Kranabetter JM, Hawkins B, Jones MD, Robbins S, Dyer T, Li T (2015a) Species turnover (β diversity) in ectomycorrhizal fungi linked to NH4 + uptake capacity. Mol Ecol 24:5992–6005
Kranabetter JM, Stoehr M, O’Neill GA (2015b) Ectomycorrhizal fungal maladaptation and growth reductions associated with assisted migration of Douglas-fir. New Phytol 206:1135–1144
Kranabetter JM, Wylie T (1998) Ectomycorrhizal community structure across forest openings on naturally regenerated western hemlock seedlings. Can J Bot 76:189–196
Kummel M, Lostroh P (2011) Altering light availability to the plant host determined the identity of the dominant ectomycorrhizal fungal partners and mediated mycorrhizal effects on plant growth. Botany 89:439–450
Leberecht M, Dannenmann M, Gschwendtner S, Bilela S, Meier R, Simon J, Rennenberg H, Schloter M, Polle A (2015) Ectomycorrhizal communities on the roots of two beech (Fagus sylvatica) populations from contrasting climates differ in nitrogen acquisition in a common environment. Appl Environ Microbiol 81:5957–5967
LeDuc SD, Lilleskov EA, Horton TR, Rothstein DE (2013) Ectomycorrhizal fungal succession coincides with shifts in organic nitrogen availability and canopy closure in post-wildfire jack pine forests. Oecologia 172:257–269
Lilleskov EA, Fahey TJ, Horton TR, Lovett GM (2002) Belowground ectomycorrhizal fungal community change over a nitrogen deposition gradient in Alaska. Ecology 83:104–115
Lilleskov EA, Hobbie EA, Horton TR (2011) Conservation of ectomycorrhizal fungi: exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal Ecol 4:174–183
Lindahl BD, Tunlid A (2015) Ectomycorrhizal fungi - potential organic matter decomposers, yet not saprotrophs. New Phytol 205:1443–1447
Logan M (2010) Biostatistical design and analysis using R. A practical guide. Wiley-Blackwell, Chichester
Mah K, Tackaberry LE, Egger KB, Massicotte HB (2001) The impacts of broadcast burning after clear-cutting on the diversity of ectomycorrhizal fungi associated with hybrid spruce seedlings in Central British Columbia. Can J For Res 31:224–235
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach
Millard P, Grelet GA (2010) Nitrogen storage and remobilization by trees: ecophysiological relevance in a changing world. Tree Physiol 30:1083–1095
Moeller HV, Peay KG, Fukami T (2014) Ectomycorrhizal fungal traits reflect environmental conditions along a coastal California edaphic gradient. FEMS Microbiol Ecol 87:797–806
Mosca E, Montecchio L, Scattolin L, Garbaye J (2007) Enzymatic activities of three ectomycorrhizal types of Quercus robur L. in relation to tree decline and thinning. Soil Biol Biochem 39:2897–2904
Münzenberger B, Golldack J, Ullrich A, Schmincke B, Hüttl RF (2004) Abundance, diversity, and vitality of mycorrhizae of scots pine (Pinus sylvestris L.) in lignite recultivation sites. Mycorrhiza 14:193–202
Nilsson RH, Veldre V, Hartmann M, Unterseher M, Amend A, Bergsten J, Kristiansson E, Ryberg M, Jumpponen A, Abarenkov K (2010) An open source software package for automated extraction of ITS1 and ITS2 from fungal ITS sequences for use in high-throughput community assays and molecular ecology. Fungal Ecol 3:284–287
Peay KG, Schubert MG, Nguyen NH, Bruns TD (2012) Measuring ectomycorrhizal fungal dispersal: macroecological patterns driven by microscopic propagules. Mol Ecol 21:4122–4136
Pena R, Tejeddor J, Zeller B, Dannenmann M, Polle A (2013) Interspecific temporal and spatial differences in the acquisition of litter-derived nitrogen by ectomycorrhizal fungal assemblages. New Phytol 199:520–528
Peršoh D (2015) Plant-associated fungal communities in the light of meta’omics. Fungal Divers 75:1–25
Phillips LA, Ward V, Jones MD (2014) Ectomycorrhizal fungi contribute to soil organic matter cycling in sub-boreal forests. ISME J 8:699–713
Pickles BJ, Twieg BD, O’Neill GA, Mohn WW, Simard SW (2015) Local adaptation in migrated interior Douglas-fir seedlings is mediated by ectomycorrhizas and other soil factors. New Phytol 207:858–871
Pritsch K, Raidl S, Marksteiner E, Blaschke H, Agerer R, Schloter M, Martmann A (2004) A rapid and highly sensitive method for measuring enzyme activities in single mycorrhizal tips using 4-methylumbelliferone-labelled fluorogenic substrates in a microplate system. J Microbiol Meth 58:233–241
R Core Team (2013) R: a language and environment of statistical computing. R foundation for statistical computing. http://www.R-project.org/. Accessed 27 Jun 2014
Rincón A, Santamaria BP, Ocana L, Verdu M (2014) Structure and phylogenic diversity of post-fire ectomycorrhizal communities of maritime pine. Mycorrhiza 24:131–141
Rineau F, Garbaye J (2009) Does forest liming impact the enzymatic profiles of ectomycorrhizal communities through specialized fungal symbionts? Mycorrhiza 19:493–500
Rineau F, Stas J, Nguyen NH, Kuyper TW, Carleer R, Vangronsveld J, Colpaert JV, Kennedy PG (2016) Ectomycorrhizal fungal protein degradation ability predicted by soil organic nitrogen availability. Appl Environ Microbiol 82:1391–1400
Root HT, McGee GG, Nyland RD (2007) Effects of two silvicultural regimes with large tree retention on epiphytic macrolichen communities in Adirondack northern hardwoods, New York, USA. Can J For Res 37:1854–1866
Simard SW, Jones MD, Durall DM (2003) Carbon and nutrient fluxes within and between mycorrhizal plants. In: Sanders IR, van der Heijden M (eds) Mycorrhizal ecology. Springer, Berlin, pp. 33–74
Smith JE, Molina R, Huso MMP, Larsen MJ (2000) Occurrence of Piloderma fallax in young, rotation-age, and old-growth stands of Douglas-fir (Pseudotsuga menziesii) in the Cascade Range of Oregon, U.S.A. Can J Bot 78:995–1001
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic Press, Cambridge
Spake R, Ezard THG, Martin PA, Newton AC, Doncaster CP (2015) A meta-analysis of functional group responses to forest recovery outside of the tropics. Conserv Biol 29:1695–1703
Talbot JM, Martin F, Kohler A, Henrissat B, Peay KG (2015) Functional guild classification predicts the enzymatic role of fungi in litter and soil biogeochemistry. Soil Biol Biochem 88:441–456
Taylor DL, Bruns TD (1999) Community structure of ectomycorrhizal fungi in a Pinus muricata forest: minimal overlap between the mature forest and resistant propagule communities. Mol Ecol 8:1837–1850
Twieg BD, Durall DM, Simard SW (2007) Ectomycorrhizal fungal succession in mixed temperate forests. New Phytol 176:437–447
van Aarle IM, Plassard C (2010) Spatial distribution of phosphatase activity associated with ectomycorrhizal plants in related to soil type. Soil Biol Biochem 42:324–330
Walker JKM, Jones MD (2013) Little evidence for niche partitioning among ectomycorrhizal fungi on spruce seedlings planted in decayed wood versus mineral soil microsites. Oecologia 173:1499–1511
Walker JKM, Ward V, Jones MD (2016) Ectomycorrhizal fungal exoenzyme activity differs on spruce seedlings planted in forests versus clearcuts. Trees–Struc Funct 30:497–508
Wallander H, Johansson U, Sterkenburg E, Durling MB, Lindahl BD (2010) Production of ectomycorrhizal mycelium peaks during canopy closure in Norway spruce forests. New Phytol 187:1124–1134
Welc M, Frossard E, Egli S, Bünemann EK, Jansa J (2014) Rhizosphere fungal assemblages and soil enzymatic activities in a 110-years alpine chronosequence. Soil Biol Biochem 74:21–30
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp. 315–322
Acknowledgments
This research was funded by a Natural Sciences and Engineering Research Council of Canada Discovery Grant to MJ. The authors are very grateful for technical and field assistance from Clive Dawson, Aaron Godin, Monika Gorzlak, Stéphane LeBihan, Logan Markaroff, Sheri Maxwell, Brian Pickles, Enav Shalev, Valerie Ward, Shayle Weibe, and Matthew Whiteside. Daniel Durall and John Klironomos provided helpful suggestions during the design of this study. Jason Pither provided statistical advice. The manuscript benefitted from the input of several anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 515 kb.)
Rights and permissions
About this article
Cite this article
Nicholson, B.A., Jones, M.D. Early-successional ectomycorrhizal fungi effectively support extracellular enzyme activities and seedling nitrogen accumulation in mature forests. Mycorrhiza 27, 247–260 (2017). https://doi.org/10.1007/s00572-016-0747-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-016-0747-7