Abstract
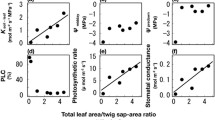
Trees drought responses could be developed in the short- or in the long-term, aiming at sustaining carbon fixation and water use efficiency (WUE). The objective of this study was to examine short- and long-term adjustments occurring in different size Pinus ponderosa Dougl. ex P. & C. Laws trees in response to seasonal drought when they are growing under different competition level. The following variables were studied: branch and stem hydraulic conductivity, canopy and stomatal conductance (gc, gs), transpiration (E), photosynthesis (A max), wood δ13C (as a proxy of intrinsic WUE), leaf to sapwood area ratio (A L:A s) and growth in the biggest (B) and the smallest (S) trees of high (H) and low (L) density stands. A L:A s was positively correlated with tree size and negatively correlated with competition level, increasing leaf hydraulic conductance in H trees. Accordingly, higher gc and E per unit A L were found in H than in L trees when soil water availability was high, but decreased abruptly during dry periods. BL trees maintained stable gc and E values even during the summer drought. The functional adjustments observed in H trees allow them to maintain their hydraulic integrity (no apparent k s losses), but their stem and leaf growth were severely affected by drought events. iWUE was similar between all tree groups in a wet season, whereas it significantly decreased in SH trees in a dry season suggesting that when radiation and water are co-limiting gas exchange, functional adjustments not only affect absolute growth, but also WUE.






Similar content being viewed by others
References
Borghetti M, Cinnirella S, Magnani F, Saracino A (1998) Impact of long-term drought on xylem embolism and growth in Pinus halepensis Mill. Trees 12:187–195
Callaway RM, DeLucia EH, Schlesinger WH (1994) Biomass allocation of montane and desert ponderosa pine: an analog for response to climate change. Ecology 75:1474–1481
Cregg BM, Olivas-García M, Hennessey TC (2000) Provenance variation in carbon isotope discrimination of mature ponderosa pine trees at two locations in the Great Plains. C J For Res 30:428–439
Curtis RO (1982) A simple index of stand density for douglas-fir. Forensic Sci 28:92–94
Fernández ME, Gyenge JE, Graciano C, Varela S, Dalla Salda G (2010) Conductancia y conductividad hidráulica. In: Fernández ME, Gyenge J (eds) Técnicas en Medición en ecofisiología vegetal, conceptos y procedimientos. Ediciones INTA, Buenos Aires, pp 53–68
Fernández ME, JE Gyenge, MM de Urquiza, S Varela (2012) Adaptability to climate change in forestry species: drought effects on growth and wood anatomy of ponderosa pines growing at different competition levels. For Syst (in press)
Fischer DG, Kolb TE, DeWald LE (2002) Changes in whole-tree water relations during ontogeny of Pinus flexilis and Pinus ponderosa in a high-elevation meadows. Tree Physiol 22:675–685
Granier A (1985) Une nouvelle méthode pour la mesure du flux de sève brute dans le tronc des arbres. Ann Sci For 42:193–200
Gyenge JE, Fernández ME, Schlichter TM (2003) Water relations of ponderosa pines in Patagonia Argentina: implications for local water resources and individual growth. Trees 17:417–423
Gyenge JE, Fernández ME, Schlichter TM (2009) Effect of pruning on branch production and water relations in widely spaced ponderosa pines. Agrof Syst 77:223–235
Hoefs J, Schidlowski M (1967) Carbon isotope composition of carbonaceous matter from the Precambrian of the Wirwatersrand system. Science 155:1096–1098
Li M, Zhu J (2011) Variation of δ13C of wood and foliage with canopy height differs between evergreen and deciduous species in a temperate forest. Plant Ecol 212:543–551
Magnani F, Grace J, Borghetti M (2002) Adjustment of tree structure in response to the environment under hydraulic constraints. Funct Ecol 16:385–393
Maherali H, DeLucia EH (2001) Influence of climate-driven shifts in biomass allocation on water transport and storage in ponderosa pine. Oecologia 129:481–491
McDowell NG, Barnard HR, Bond BJ, Hinckley TM, Hubbard RM, Ishii H, Köstner B, Magnani F, Marshall JD, Meinzer FC, Phillips N, Ryan MG, Whitehead D (2002) The relationship between tree height and leaf area: sapwood area ratio. Oecologia 132:12–20
McDowell N, Brooks JR, Fitzgerald SA, Bond BJ (2003) Carbon isotope discrimination and growth response of old Pinus ponderosa trees to stand density reductions. Plant Cell Environ 26:631–644
McDowell NG, Adams HD, Bailey JD, Hess M, Kolb TE (2006) Homeostatic maintenance of ponderosa pine gas exchange in response to stand density changes. Ecol Appl 16:1164–1182
Michelot A, Eglin T, Dufrêne E, Lelarge-Trouverie C, Demesin C (2011) Comparison of seasonal variations in water-use efficiency calculated from carbon isotope composition of tree rings and flux data in a temperate forest. Plant Cell Environ 34:230–244
Monteith JL, Unsworth MH (1990) Principles of environmental physics. Edward Arnold, New York
Neter J, Waserman W (1974) Applied linear statistical models. Regression, analysis of variance and experimental design. R.D. Irwin Inc., Illinois
Ogle K, Pacala SW (2009) A modeling framework for inferring tree growth and allocation from physiological, morphological and allometric traits. Tree Physiol 29:587–605
Oren R, Sperry JS, Katul GG, Pataki DE, Ewers BE, Phillips N, Schäfer KVR (1999) Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plan Cell Environ 22:1515–1526
Panarello HO (1987) Relaciones entre isótopos de elementos livianos para estudiar procesos ambientales y paleotemperaturas. PhD thesis, Universidad de Buenos Aires, FCEN, Buenos Aires, Argentina, p 105
Piñol J, Sala A (2000) Ecological implications of xylem embolism for several Pinaceae in the Pacific Northern USA. Funct Ecol 14:538–545
Ryan MG, Bond BJ, Law BE, Hubbard RM, Woodruff D, Cienciala E, Kucera J (2000) Transpiration and whole-tree conductance in ponderosa pine trees of different heights. Oecologia 124:553–560
Simonin K, Kolb TE, Montes-Helu M, Koch GW (2006) Restoration thinning and influence of tree size and leaf area to sapwood area ratio on water relations of Pinus ponderosa. Tree Physiol 26:493–503
Skov KR, Kolb TE, Wallin KF (2004) Tree size and drought affect ponderosa pine physiological response to thinning and burning treatment. Forensic Sci 50:81–91
Spicer R, Gartner BL (1998) How does a gymnosperm branch (Pseudotsuga menziesii) assume the hydraulic status of a main stem when it takes over as leader? Plant Cell Environ 21:1063–1070
Warren CR, McGrath JF, Adams MA (2001) Water availability and carbon isotope discrimination in conifers. Oecologia 127:476–486
Whitehead D, Jarvis PG (1981) Coniferous forest and plantations. In: Kozlowski TT (ed) Water deficits and plant growth. Academic Press, New York, pp 49–152
Whitehead D, Edwards WRN, Jarvis PG (1984) Conducting sapwood area, foliage area, and permeability in mature tree of Picea sitchensis and Pinus contorta. Can J For Res 14:940–947
Acknowledgments
The authors wish to thank INTA (National Institute for Agricultural Technology of Argentina) and CONICET (National Council for Scientific and Technical Research of Argentina) for providing the financial support through PNFOR042131 and PNFOR042141 and PIP 1122008010239101, respectively. We gratefully acknowledge the Martin family for allowing us to carry out our research on their property.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Grams.
Appendix 1
Appendix 1
Canopy conductance
Canopy conductance (gc, mm s−1) was estimated according to Monteith and Unsworth (1990) as:
where γ(T) is the psychrometric constant (as a function of temperature T, kPa K−1), λ(T) is the latent heat of vaporization of water (J kg−1), E is transpiration expressed on A L basis (kg m−2s−1), C p is the specific heat of air, ρ(T) is the density of the air (kg m−3), and ΔW is the leaf-to-air vapor pressure difference (or gradient, kPa), assuming that leaf temperature was the same as air temperature.
Sensitivity of stomata to VPD
It was estimated following Oren et al. (1999) as:
where m is the stomatal sensitivity to VPD (mm s−1 ln(kPa)−1) and b the reference gc at VPD = 1 kPa (mm s−1). Parameters were estimated using least squares regression analysis. No lag time between sap flux data and VPD was observed, as was also reported in Fischer et al. (2002).
Specific conductivity of branch wood
Specific conductivity (k s, kg m−1 MPa−1 s−1) is a measure of the hydraulic efficiency of a unit of xylem and is defined by Darcy′s law as:
where Q is the volume flow rate (m3s−1), l is the length of the segment (m), and ΔP is the pressure difference between the two ends of the segment (MPa). All conductivity calculations were corrected to 20 °C to account for changes in fluid viscosity with temperature (Spicer and Gartner 1998). To determine k s of branch wood, single branches (0.5–1 m long) from five trees growing at each density plot were excised in the afternoon (maximum VPD—maximum loss of k s), sprayed with water and wrapped in plastic bags to avoid desiccation and the possibility of further k s reduction by dehydration. In the laboratory, a segment from the middle of the branch was cut under water to avoid new embolisms and attached to the end of a transparent plastic hose. A 1-ml graduated pipette was attached to the other end of the hose, located 1 m above the branch sample. Volume flow rate was measured by timing the movement of the meniscus across 0.1 ml graduations. Repeated measurements on the same sample showed that direction of flow did not affect results.
Volume fraction of water in the xylem of the branch
Volume fraction of water (V w) was determined in 4–5 cm long barked twig segments as:
where W f and W d are the fresh and dry weight of segments, V f is fresh volume (estimated from the length and the average diameter of both ends), ρ w is the density of water. Dry weight was determined after oven-drying the samples at 80 °C for 48 h (see Borghetti et al. 1998). Sample weight was measured to the nearest 0.1 mg.
Whole tree plant liquid phase hydraulic conductance
Whole tree plant liquid phase hydraulic conductance (Kh, ml min−1 MPa−1) was estimated as:
where ψsoil is the soil water potential (estimated from ψpd) and E is the average transpiration estimated from 14:00 to 19:00 h taking into account the radial sapflow decrease (Gyenge et al. 2003). Calculation of Kh was limited to those dates in which water potential was measured.
Rights and permissions
About this article
Cite this article
Gyenge, J., Fernández, M.E. & Varela, S. Short- and long-term responses to seasonal drought in ponderosa pines growing at different plantation densities in Patagonia, South America. Trees 26, 1905–1917 (2012). https://doi.org/10.1007/s00468-012-0759-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-012-0759-7