Abstract
Ischaemia–reperfusion (IR) injury is a composite of the injury sustained during a period of reduced or absent blood flow to a tissue or organ and the additional insult sustained upon reperfusion that limits the amount of tissue that can be salvaged. IR injury plays a central role in both native and transplant acute kidney injury (AKI). Native AKI is associated with increased morbidity and mortality in hospital inpatients, and transplant AKI contributes to graft dysfunction, ultimately limiting graft longevity. In this review, we discuss the potential therapeutic benefits of a cost-effective and low-risk intervention, remote ischaemic preconditioning (RIPC), and its applicability in the prevention and reduction of AKI.
Similar content being viewed by others
Introduction
When an organ or tissue is rendered ischaemic, there is inevitable cell death and tissue injury, the extent of which can be limited by timely reperfusion. However, paradoxically, an additional injury occurs upon reperfusion which limits the amount of tissue that can be salvaged. This composite injury is termed ‘ischaemia–reperfusion (IR) injury’.
In both native [1–3] and kidney transplant [4] acute kidney injury (AKI), IR injury plays a significant role. The pathogenesis of AKI, regardless of aetiology, results in a degree of IR injury, the majority of which affects the vulnerable tubular epithelium. Native AKI is associated with the subsequent development of chronic kidney disease (CKD) [5, 6] and increased mortality in patients admitted to hospital [7]. In renal transplantation, despite an improvement in 1-year kidney transplant survival, long-term allograft survival has not altered significantly in recent years [8–10]. IR injury at the time of transplantation is associated with an increased risk of acute rejection, delayed graft function and poor overall graft function [11]. Therefore, strategies to reduce IR injury at the time of transplantation may be the best therapeutic intervention to increase graft longevity.
Ischaemic preconditioning is an intervention targeted against IR injury whereby brief, non-lethal periods of ischaemia activate an innate response that confers protection against a later prolonged and thus potentially lethal period of ischaemia. Ischaemic conditioning may be applied before (preconditioning), during (perconditioning) or following (postconditioning) ischaemia and may be applied remotely. In this review, we discuss the potential of remote ischaemic preconditioning (RIPC) in protecting against native and transplant AKI. The therapeutic potential of this simple, low-cost protective strategy has attracted much attention in recent years, with a number of large clinical trials about to report their findings, including one in renal transplantation.
Ischaemia–reperfusion injury
Ischaemia–reperfusion injury was first described in 1960 by Jennings et al. who demonstrated that myocardial infarct size in the dog following 24 h of ischaemia alone was similar to that after 30 min of ischaemia followed by 60 min of reperfusion [12]. A variety of experimental interventions to specifically modify the reperfusion phase of IR have been shown to reduce IR injury, further establishing the injurious nature of reperfusion.
Strategies to limit clinical ischaemic injury have mainly focussed on timely reperfusion, including interventions such as primary coronary intervention, thrombolysis for stroke and reduction of both warm and cold ischaemic times in transplantation. However, given that a significant proportion of tissue dysfunction and cell death following IR injury is attributable to reperfusion injury and that there has arguably been maximal optimisation of therapeutic techniques and their timing (within the current framework of healthcare delivery), attention has turned towards interventions which specifically target IR injury, either to enhance resistance to ischaemia and/or to reduce reperfusion injury. One such strategy is ischaemic preconditioning.
Ischaemic preconditioning
Ischaemic preconditioning is a whole body innate reflex that protects against subsequent IR injury and is activated by brief, non-lethal periods of tissue or organ ischaemia. The phenomenon was first described in 1986 by Murray et al. who demonstrated that a series of 45-min periods of circumflex coronary artery occlusion, separated by 5 min of reperfusion, could significantly reduce myocardial infarct size in dogs following subsequent prolonged ischaemia [13]. The magnitude of the effect size observed was much greater than that found with any pharmacological agent, and the effect was widely reproducible and subsequently demonstrated in many different animal models and species, including chicken, pig, rat, dog, mouse and sheep [14].
Following on from Murray et al.’s [13] discovery, Pryzlenk et al. demonstrated that ischaemic conditioning could be applied remotely [15]. These authors found that brief periods of ischaemia applied to one vascular bed could remotely protect another—in this case circumflex artery preconditioning protected the anterior descending coronary artery territory from injury following a subsequent prolonged occlusion [15]. Subsequent studies established that the preconditioning stimulus could be applied to a different organ, with protection spreading to remote organs, including the heart, brain and kidney. Now termed ‘remote ischaemic preconditioning’, this intervention gained potential clinical applicability with the discovery that the ischaemic preconditioning stimulus could be applied non-invasively in humans using a blood pressure cuff placed on a limb and inflated above systolic blood pressure to induce limb ischaemia [16].
The protective effects of ischaemic preconditioning have been demonstrated to occur in two ‘windows’, with the initial period of protection occurring immediately following the preconditioning stimulus and lasting for between 1 and 4 h [17–19], and the onset of a delayed or ‘second window of protection’ occurring at 24 h following preconditioning, and lasting for between 24 and 72 h [18, 20, 14]. However, it should be noted that timely reperfusion remains a requirement even despite ischaemic preconditioning [13], with the latter delaying rather than abrogating the onset of cellular death. The discovery that preconditioning could be activated both non-invasively and remotely led directly to potential clinical applications of this therapy. Initial interest in cardioprotection has extended to virtually all organ systems that may be subjected to IR injury in the clinical setting, including not only cerebrovascular disease but also many forms of surgery.
Mechanisms of protection of RIPC
From its inception, researchers have postulated that RIPC relies on three components: local mediators which initiate (or trigger) the preconditioning cascade, humoral and/or neural factors which transfer protection systemically from the remote site and end-effectors which confer this protection to the threatened organ or tissue.
Triggers
Certain factors, termed ‘trigger factors’, are released locally at the time of preconditioning ischaemia and include adenosine, bradykinin and endogenous opioids. These triggers initiate the cascade of protection locally by activating G-protein-coupled receptors [14] and thereby promoting the recruitment of protein kinase mediators (such as PI3K, ERK/MAPK, PKC and JAK/STAT) [21–23].
Signal transduction
The mechanism by which the protective signal is transferred systemically from the area of index ischaemia has been the subject of some debate. Evidence for the involvement of a humoral factor is supported by the observation that protection can be transferred by the transfusion of serum from a rabbit that has undergone ischaemic preconditioning to one which has not [24, 25]. This factor is believed to be a protein that is heat stable, dialysable and of a size less than 15 kDa [26, 27]. In pigs, RIPC applied to the recipient animal conferred protection against IR injury to the denervated donor heart during transplantation, again supporting a humoral hypothesis [28]. Attempts to identify this circulating factor have proved challenging. However, recently, stromal cell-derived factor-1 (SDF-1α or CXCL12), a cardioprotective chemokine of 10 kDa that is induced by hypoxia, has been demonstrated to be upregulated following RIPC in rats. The resultant cardioprotection was blocked in rats treated with AMD3100, a highly specific inhibitor of CXCR4, the target receptor for SDF-1α [29].
Neurogenic mechanisms of signal transfer have also been suggested. In rats, Dong et al. demonstrated that femoral nerve section abolished the effects of limb IPC [30]. Local injection of adenosine into the nerve produced a similar protection to that of IPC, whereas intravenous injection of adenosine had no effect. Administration of an adenosine antagonist partially abolished the effects of IPC [30]. In a rat myocardial infarction model, hexamethonium (an autonomic antagonist) abolished the protection by RIPC achieved by mesenteric artery occlusion [31]. The autonomic ganglion blocker trimetaphan has been shown to inhibit RIPC in a human model [19].
The humoral and neuronal pathways may work in series to spread protection systemically. Lim et al. demonstrated that in mice, femoral vein occlusion or femoral and sciatic nerve resection abolished the protective effects of RIPC, implicating both humoral and neural pathways [32].
End-effectors
Ischaemic preconditioning activates at least three main salutatory pathways, i.e. the cyclic guanosine monophosphate/cGMP-dependent protein kinase (cGMP/PKG) pathway [33], the reperfusion injury salvage kinase (RISK) pathway [34, 35] and the survivor-activating factor enhancement (SAFE) pathway [36]. There is a degree of overlap between these pathways, in particular where they converge in mitochondria [37]. In the mitochondria, although there is some uncertainty regarding the mechanism, the potassium-dependent ATP (KATP) channel is activated, leading to closure of the mitochondrial permeability transition pore (mPTP). Closure of the mPTP prevents the influx of ions through this channel, thus preventing mitochondrial rupture and cell death by apoptosis.
The second window of protection—delayed or late IPC
Ischaemic preconditioning initiates a complex genomic and proteomic response that is thought to underpin the late phase of protection. This includes regulation of anti-apoptotic and anti-inflammatory gene transcription, which is likely to be responsible for the second window of protection [38, 39]. Later phase protection requires the synthesis of inducible nitric oxide synthase, heat shock proteins or cyclo-oxygenase-2, secondary to the upregulation of genes for these factors. These then act locally via the mPTP or KATP channels to induce a state of protection [40].
The role of the innate immune system
Activation of the innate immune system by non-lethal periods of ischaemia may be mediated by germline-encoded toll-like receptors (TLRs) and may contribute to the development of ischaemic tolerance. Activation of feedback inhibitors of inflammation following an ischaemic insult effectively renders the tissue immunosuppressed; consequently, the inflammatory response to a subsequent lethal insult is attenuated [41]. In mice, deficiency of TLR4 is associated with a reduction in myocardial IR injury in mice [42], but the absence of a functional TLR4 has been demonstrated to block IPC [43, 44].
Pathophysiology of renal IR injury
During ischaemia, renal epithelial cells are deprived of ATP and are therefore unable to maintain essential homeostatic processes. This ultimately leads to cell death by apoptosis or necrosis [45] if timely reperfusion does not occur. Although any segment of the nephron may be affected, the cells most vulnerable are in the renal proximal tubule and distal medullary thick ascending limb of the loop of Henle [46, 47]. The factors contributing to the vulnerability of these cells to ischaemia are high metabolic rate, required for ion transport, and a limited capacity for anaerobic metabolism. Additionally, marked microvascular congestion and hypoperfusion have been found in this region which persists despite restoration of cortical blood flow, therefore contributing to prolonged ischaemic injury [47]. Proximal tubular cell injury leads to afferent arteriolar vasoconstriction by tubuloglomerular feedback, luminal obstruction and backleak of filtrate across injured cells, with resultant ineffective glomerular filtration and a profound drop in the glomerular filtration rate (GFR). Endothelial cell injury and endothelial dysfunction are primarily responsible for this phenomenon, known as the extension phase of AKI [48].
Ischaemic injury results in the loss of the apical brush border of proximal tubular cells. Disrupted microvilli detach from the apical surface, forming membrane bound blebs that are released into the tubular lumen. The detachment and loss of tubular cells, in combination with brush border vesicle remnants, cellular debris and uromodulin, result in tubular casts which may cause obstruction [3, 47]. Necrotic cell death is rare, but it may occur in the highly susceptible outer medullary regions. Conversely, apoptosis may be seen in both proximal and distal tubular cells. Apoptosis has been demonstrated in distal tubular cells during nephrotoxic AKI and also in donor kidneys before transplantation—in one study this was found to be associated with delayed graft function [49]. In addition to tubular cell injury, podocyte dysfunction may occur, with foot process effacement and loss of slit diaphragm integrity, and resultant proteinuria [50].
Alteration in the cell cytoskeleton also contributes to injury during ischaemia and is of particular importance in the specialised cells of the proximal tubule in which the cell membrane is augmented by microvilli, which are essential to the normal functioning of these cells. The spectrin–actin cytoskeleton is responsible for the adherence of ion pumps to the cell membrane, and cytoskeletal disruption leads to the redistribution of basolateral Na+/K+ATPase pumps to the apical membrane within 10 min of cytoskeletal disruption. The resultant bidirectional transport of sodium and water across the apical and basolateral cellular membrane leads to cellular sodium being retransported to the tubular lumen, and thus to an increased fractional excretion of sodium. Effective sodium transport is further disrupted by ineffective transcellular sodium transport, secondary to a deficiency in the supply of ATP. The high concentration of sodium in the filtrate activates glomerular feedback, stimulating the macula densa to induce afferent arteriolar vasoconstriction, with a subsequent reduction in GFR [47]. A diagram summarising the effects of IR injury on the renal tubular epithelium is shown in Fig. 1.
In renal transplants, histological abnormalities associated with delayed graft function due to prolonged ischaemia include increased brush border loss, tubular necrosis, cell shedding, tubular dilatation and interstitial inflammation [51]. The appearance of mild histological abnormalities is often associated with substantial effects on graft function.
Renal dendritic cells and macrophages play an important role in the innate and adaptive immune response in acute IR injury [52] and are also thought to contribute to injury through the production of tumour necrosis factor [53]. Animal models of renal IR injury have shown increased levels of mannin–binding lectin (MBL) early in reperfusion, with subsequent complement deposition [54], although other, more recent animal studies have demonstrated that following reperfusion, the internalisation of MBL by tubular epithelial cells promotes cell death independently of complement activation [55]. Activation of the innate immune system by germline-encoded TLRs in response to renal ischaemia contributes to inflammation and proximal tubular injury, and resolution of this inflammation might be causal in the recovery of injured tubular cells [56, 57]. Upregulation of gene and protein expression of TLR2 and TLR4 in rat kidneys has been demonstrated following IR injury [58] and may be of particular importance to AKI in kidney transplantation.
Evidence for a clinical benefit of RIPC
Most human studies have used limb ischaemia to activate RIPC due to the inaccessibility of vital organs for IPC. The first such clinical study demonstrated an effect of limb ischaemia to prevent experimental IR injury to the endothelium and was rapidly followed by the first clinical trial of RIPC [59]. In this small study, eight patients undergoing coronary artery bypass grafting (CABG) were randomised to receive either RIPC or control. The results demonstrated an increase in blood lactate dehydrogenase (collected from the coronary perfusion catheter) in the preconditioned group, which the investigators attributed to an ability to maintain anaerobic metabolism in preconditioned cells [59].
In 2007, Hausenloy et al. were the first to demonstrate a reduction in troponin T levels in adults randomised to receive RIPC prior to CABG with cross-clamp fibrillation [60]. In 2009, Venugopal et al. also demonstrated a reduction in troponin T following RIPC in patients undergoing cold blood cardioplegia [61]. However in 2010, Rahman et al. published a larger single-centre double-blind randomised controlled trial in which 162 patients undergoing CABG were randomised to receive either RIPC or placebo. In this study there was no difference in troponin release or in any other clinical outcome between the two groups [49]. Most recently, a larger single-centre study of 329 patients undergoing isolated CABG with cold blood cardioplegia and cardiopulmonary bypass, randomised to RIPC or placebo, demonstrated a reduction in post-operative troponin I in the preconditioned group [62]. The authors also attempted to address the question of whether a reduction in troponin equated to a measurable longer term clinical benefit. They reported a reduction in all-cause mortality in the preconditioned group that was sustained during >4 years of follow-up.
In the clinical setting of primary coronary intervention (PCI) for acute myocardial infarction, Iliodromitis et al. were the first to investigate whether RIPC would attenuate the inflammatory response in elective single vessel PCI with coronary stenting. In their 2006 study, these authors demonstrated an increase in cardiac enzymes and C-reactive protein in the preconditioned group and postulated that RIPC increased the inflammatory response [63]. Subsequently, Hoole et al., in their 2009 study of 242 patients undergoing elective PCI, demonstrated that RIPC prior to PCI attenuated procedure-related troponin release [52]. However, in a separate study, the same group showed that there was no beneficial effect on left ventricular dysfunction during coronary balloon occlusion in single vessel coronary disease [53].
Increased interest in the clinical usefulness of RIPC in the setting of myocardial ischaemia (CABG or PCI) has led to the publication of many other small trials in recent years, all reporting differing outcomes. However, the largest study to date by far—a multicentre double-blind randomised controlled trial, ‘Effect of Remote Ischemic preConditioning on clinical outcomes in patients undergoing Coronary Artery bypass graft surgery’ (ERICCA), is currently underway to investigate whether RIPC improves 1-year cardiovascular outcomes and reduces AKI in the setting of CABG. This trial has recently completed recruitment of 1,610 patients, randomised to either RIPC or sham-RIPC. The primary endpoint is MACCE (=major adverse cardiac and cerebral event(s)—a combined outcome score) at 1 year, but quality of life and echocardiography follow-up are also included [64].
Evidence for a clinical benefit of RIPC in the kidney
In AKI, there are several interesting clinical questions. Firstly, can RIPC protect the kidneys against ‘bystander’ AKI, i.e. does RIPC prior to myocardial injury reduce collateral damage to the kidney? Secondly, can RIPC protect against planned ischaemic insults such as contrast nephropathy? Thirdly, can RIPC reduce IR injury to the allograft during transplantation? Additionally, can RIPC protect patients with chronic or end-stage kidney disease against IR injury in other organ systems, for example myocardial stunning during haemodialysis?
Animal studies have demonstrated the therapeutic potential of RIPC in protecting from IR injury in the kidney [65, 66], but these benefits have proved difficult to translate into clinical studies in humans. Although several studies have been published in humans, these tend to be small, single-centre studies, and many report differing and short-term endpoints, thus making them difficult to compare or interpret. In addition, the role of co-existent comorbid states and polypharmacy in such patients are confounders, and the degree to which cannot easily be ascertained. A summary of the currently available clinical trial evidence is provided in Table 1, which also presents details on the published trials of RIPC that report renal endpoints.
Clinical trials of RIPC to protect against bystander AKI
A recent meta-analysis of studies in cardiac/abdominal aortic aneurysm surgery suggests that there is a benefit of RIPC in reducing renal injury post-surgery [67]. However in only five trials were the absolute creatinine values documented and included in the analyses; however, differing measures were reported and so the results were adjusted and reported as standardised mean values. Additionally, these trials were not powered towards renal endpoints, and the total number of patients included was 377, which is still most likely underpowered to detect a significant renal effect. The doubt over whether RIPC can protect against bystander renal injury during surgery should be answered in due course by currently ongoing large clinical trials, such as ERICCA.
RIPC in protection against contrast nephropathy
One other potential application that has been investigated in a clinical trial is the use of RIPC to protect against contrast-induced AKI. Patients with pre-existing renal dysfunction (serum creatinine >1.4 mg/dl or estimated (e) GFR of <60 ml/min/1.73 m2) were randomised to receive RIPC (4 × 5-min arm cuff inflations) or sham prior to elective coronary angioplasty. The authors reported a reduction in the rate of contrast-induced AKI, from 40 % in the control group to 12 % in the RIPC group (n = 100, p = 0.002) [68]. Not only are these results of potential relevance in the setting of CKD patients undergoing routine investigation or elective angiography, especially in avoiding the precipitation of dialysis dependence in patients with CKD stage 5 estimated GFR [(eGFR) <15 ml/min], but they may also help to relieve anxiety surrounding the use of PCI in patients presenting acutely. Studies have demonstrated increased morbidity and mortality in patients with CKD who present with acute coronary syndromes, some of which may be attributable to management possibly being compromised by a reluctance to use therapies involving contrast, which may subsequently precipitate dialysis [69].
A further single-centre randomised controlled trial, ‘Effect of Remote Ischaemic Conditioning on Contrast-Induced Nephropathy in patients undergoing elective coronary angiography (ERICCIN)’, is currently underway. The aim of this study is to recruit 362 patients at risk of contrast nephropathy (pre-existing eGFR <60 ml/min/1.73 m3), randomised to either 4 cycles of 5 min of arm cuff inflation to 200 mmHg or sham (10 mmHg), administered 2 h prior to contrast administration for cardiac catheterisation. The primary endpoint is a rise in creatinine of >25 % of eGFR, or a rise in creatinine of >44 μmol/l at 48 h, with secondary endpoints of eGFR over 3 months and the biomarkers neutrophil gelatinase-associated lipocalin (NGAL) and urinary albumin at 6 h, 48 h and 3 months post-contrast administration [70].
RIPC in kidney transplantation
The use of direct IPC in transplantation (preconditioning of the donor organ at retrieval by repeated clamping/unclamping of the arterial supply) has been investigated in clinical trials in liver transplantation [71]. However, no similar studies have as yet been published in kidney transplantation.
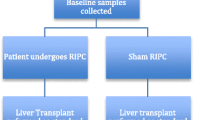
A pilot clinical trial carried out by our group in the setting of paediatric living-donor renal transplantation demonstrated the protective effects of late (‘second window’) RIPC. A blood pressure cuff was used to cause 5-min periods of limb ischaemia (3 cycles, applied to the donor and recipient) 24 h in advance of surgery. A prospective cohort of patients (n = 20) were randomised in a blinded fashion to sham RIPC or RIPC (n = 10 in each group). The groups did not differ in terms of donor/recipient age, sex, weight, height, baseline creatinine or cold ischaemic time.
Post-operative excretion of retinol binding protein (RBP; area under the curve for RBP 72 h post-transplantation) in RIPC patients was significantly reduced compared to the controls [1.2 × 105 vs. 1.5 × 105, respectively; p = 0.02]. The time for plasma creatinine to halve was shorter in the RIPC group than in the controls (5.5 ± 2.3 vs. 9.4 ± 3.5, respectively; p = 0.007). RIPC resulted in significant improvement of long-term renal function. The mean area under the curve (AUC eGFR) for the intermediate follow-up period (1–24 months post-transplantation) was 366 and 303 in the control (n = 10) and RIPC (n = 9) groups, respectively (p = 0.009). The AUC eGFR for the late follow-up period (up to 60 months post-transplantation) was also significantly higher in the RIPC group (1,190; n = 7] than in the control group (1,103; n = 7; p = 0.04) (Fig. 2).
A second randomised controlled study of RIPC in renal transplantation was published (as a letter to the editor) earlier this year. In this small study, live donor kidney transplant recipients and their donors were randomised in pairs to receive either donor RIPC, recipient RIPC or none. The RIPC stimulus was 3 × 5-min leg cuff inflations to 300 mmHg, separated by 5 min of reperfusion. The timing of the RIPC stimulus prior to surgery was not specified. In this small study, the authors did not observe any differences between the three groups in terms of urine volume, plasma creatinine, AKI biomarkers, length of hospital stay or cost [72].
Of note, a study of direct ischaemic preconditioning in the setting of deceased-donor transplantation has reported a significant improvement in creatinine, eGFR and urine NGAL in patients who received the intervention. In this study, perconditioning (i.e. ischaemic conditioning performed during the period of ischaemic injury) was delivered by 3 × 5-min cycles of external iliac artery clamping, carried out while the venous and arterial anastomoses were formed [73].
A much larger study of RIPC in live donor renal transplantation, the REnal Protection Against Ischaemia Reperfusion in transplantation (REPAIR), has finished recruiting and completed 1 year of follow-up for the primary endpoint (iohexol GFR at 12 months post-transplantation). This study is unique in that it examines whether there is an additive effect of early and late preconditioning—participants are randomised in donor/recipient pairs to receive early RIPC, late RIPC, both or none. A study of RIPC immediately prior to surgery in cadaveric renal transplantation (recipient RIPC) is also underway in Scandinavia, and the Remote Ischemic Preconditioning in Abdominal Organ Transplantation (RIPCOT) study is recruiting 580 deceased organ donors and recipients of kidneys, livers and pancreata.
It is hoped that the results of these trials will determine whether RIPC should be accepted as a standard part of care in renal transplantation. Further questions, such as whether RIPC should be applied to the donor, recipient or both, will need to be directly addressed in subsequent studies.
Other potential applications of RIPC
Other potential benefits of RIC during native AKI, such as to improve renal function, reduce mortality or protect against long-term CKD, have not as yet been investigated. It is challenging to conduct studies in this setting in that the onset, degree and nature of the IR injury cannot be predicted and, therefore, the comparison of cohorts in clinical trials would prove difficult.
A clinical trial is currently underway investigating the effects of RIPC to protect against myocardial stunning in haemodialysis patients. The primary endpoint is regional wall abnormalities on two-dimensional echocardiogram within 4 h of the intervention, with secondary endpoints including: change in haemodynamic variables, frequency of intradialytic hypotension, longer term echocardiographic changes and the biomarkers troponin-T, plasma interleukin-6 and N-type pro-brain natriuretic peptide.
Summary
Remote ischaemic preconditioning is a safe, inexpensive and well-tolerated intervention that might have significant clinical benefits in reducing tissue and organ damage following IR injury. Several large randomised controlled clinical trials are currently underway which should resolve current conflicts regarding the potential utility of this technique in clinical practice.
References
Liaño F, Pascual J (1996) Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 50:811–818
Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM (2004) Program to Improve Care in Acute Renal Disease Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 66:1613–1621
Lameire N, Van Biesen W, Vanholder R (2005) Acute renal failure. Lancet 365:417–430
Tullius SG, Reutzel-Selke A, Egermann F, Nieminen-Kelhä M, Jonas S, Bechstein WO, Volk HD, Neuhaus P (2000) Contribution of prolonged ischemia and donor age to chronic renal allograft dysfunction. J Am Soc Nephrol 11:1317–1324
Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ (2009) Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20:223–228
Murugan R, Kellum JA (2011) Acute kidney injury: what’s the prognosis? Nat Rev Nephrol 7:209–217
Liangos O, Wald R, O’Bell JW, Price L, Pereira BJ, Jaber BL (2006) Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol 1:43–51
Lodhi SA, Meier-Kriesche HU (2011) Kidney allograft survival: the long and short of it. Nephrol Dial Transplant 26:15–17
Meier‐Kriesche H, Schold JD, Kaplan B (2004) Long‐term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies. Am J Transplant 4:1289–1295
Chang SH, Russ GR, Chadban SJ, Campbell SB, McDonald SP (2007) Trends in kidney transplantation in Australia and New Zealand, 1993-2004. Transplantation 84:611–618
Mehrabi A, Mood ZA, Sadeghi M, Schmied BM, Muller SA, Welsch T, Kuttymuratov G, Wente MN, Weitz J, Zeier M, Morath C, Riediger C, Schemmer P, Encke J, Büchler MW, Schmidt J (2007) Thymoglobulin and ischemia reperfusion injury in kidney and liver transplantation. Nephrol Dial Transplant 22:viii54–viii60
Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H (1960) Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol 70:68–78
Murry C, Jennings R, Reimer K (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–36
Yellon DM, Downey JM (2003) Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 83:1113–1151
Przyklenk K, Bauer B, Ovize M, Kloner R, Whittaker P (1993) Regional ischemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 87:893–899
Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R (2002) Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 106:2881–2883
Lawson CS, Downey JM (1993) Preconditioning: state of the art myocardial protection. Cardiovasc Res 27:542–550
Baxter GF, Goma FM, Yellon DM (1997) Characterisation of the infarct-limiting effect of delayed preconditioning: timecourse and dose-dependency studies in rabbit myocardium. Basic Res Cardiol 92:159–167
Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ (2005) Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol 46:450–456
Marber M, Latchman D, Walker J, Yellon D (1993) Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation 88:1264–1272
Hausenloy DJ, Lecour S, Yellon DM (2010) Reperfusion injury salvage kinase and survivor activating factor enhancement prosurvival signaling pathways in ischemic postconditioning: two sides of the same coin. Antioxid Redox Signal 14:893–907
Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK (2011) Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol 106:135–145
Hausenloy DJ, Yellon DM (2006) Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res 70:240–253
Dickson EW, Lorbar M, Porcaro WA, Fenton RA, Reinhardt CP, Gysembergh A, Przyklenk K (1999) Rabbit heart can be “preconditioned” via transfer of coronary effluent. Am J Physiol 277:H2451–H2457
Dickson E, Reinhardt C, Renzi F, Becker R, Porcaro W, Heard S (1999) Ischaemic preconditioning may be transferable via whole blood transfusion: preliminary evidence. J Thromb Thrombolysis 8:123–129
Patel HH, Moore J, Hsu AK, Gross GJ (2002) Cardioprotection at a distance: mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. J Mol Cell Cardiol 34:1317–1323
Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, Li J, Gross G, Wilson GJ, Callahan J, Redington AN (2009) Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci 117:191–200
Konstantinov IE, Li J, Cheung MM, Shimizu M, Stokoe J, Kharbanda RK, Redington AN (2005) Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation 79:1691–1695
Davidson SM, Selvaraj P, He D, Boi-Doku C, Yellon RL, Vicencio JM, Yellon DM (2013) Remote ischaemic preconditioning involves signalling through the SDF-1α/CXCR4 signalling axis. Basic Res Cardiol 108:1–10
Dong JH, Liu YX, Ji ES, He RR (2004) Limb ischemic preconditioning reduces infarct size following myocardial ischemia-reperfusion in rats. Sheng Li Xue Bao 56:41–46
Gho BCG, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD (1996) Myocardial protection by brief ischemia in noncardiac tissue. Circulation 94:2193–2200
Lim SY, Yellon DM, Hausenloy DJ (2010) The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol 105:651–655
Burley DS, Ferdinandy P, Baxter GF (2007) Cyclic GMP and protein kinase‐G in myocardial ischaemia–reperfusion: opportunities and obstacles for survival signaling. Br J Pharmacol 152:855–869
Hausenloy DJ, Yellon DM (2004) New directions for protecting the heart against ischaemia–reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res 61:448–460
Hausenloy DJ, Yellon DM (2007) Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev 12:217–234
Lecour S (2009) Activation of the protective survivor activating factor enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? J Mol Cell Cardiol 47:32–40
Heusch G, Boengler K, Schulz R (2010) Inhibition of mitochondrial permeability transition pore opening: the holy grail of cardioprotection. Basic Res Cardiol 105:151–154
Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MMH, Cherepanov V, Downey GP, Liu PP, Cukerman E, Coles JG, Redington AN (2004) The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics 19:143–150
Murphy T, Walsh PM, Doran PP, Mulhall KJ (2010) Transcriptional responses in the adaptation to ischaemia–reperfusion injury: a study of the effect of ischaemic preconditioning in total knee arthroplasty patients. J Transl Med 8:46
Hausenloy DJ, Yellon DM (2010) The second window of preconditioning (SWOP) where are we now? Cardiovasc Drugs Ther 24:235–254
Karikó K, Weissman D, Welsh FA (2004) Inhibition of toll-like receptor and cytokine signaling-a unifying theme in ischemic tolerance. J Cereb Blood Flow Metab 24:1288–1304
Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, Browder IW, Kao RL, Williams DL, Li C (2007) Protection against myocardial ischemia/reperfusion injury in TLR4-deficient mice is mediated through a phosphoinositide 3-kinase-dependent mechanism. J Immunol 178:7317–7324
Wang F, Birch SE, He R, Tawadros P, Szaszi K, Kapus A, Rotstein OD (2010) Remote ischemic preconditioning by hindlimb occlusion prevents liver ischemic/reperfusion injury: the role of High Mobility Group-Box 1. Ann Surg 251:292–299
Pradillo JM, Fernández-López D, García-Yébenes I, Sobrado M, Hurtado O, Moro MA, Lizasoain I (2009) Toll-like receptor 4 is involved in neuroprotection afforded by ischemic preconditioning. J Neurochem 109:287–294
Lien Y-HH, Lai L-W, Silva AL (2003) Pathogenesis of renal ischemia/reperfusion injury: lessons from knockout mice. Life Sci 74:543–552
Munshi R, Hsu C, Himmelfarb J (2011) Advances in understanding ischemic acute kidney injury. BMC Med 9:11
Sharfuddin AA, Molitoris BA (2011) Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7:189–200
Molitoris BA, Sutton TA (2004) Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int 66:496–499
Oberbauer R, Rohrmoser M, Regele H, Mühlbacher F, Mayer G (1999) Apoptosis of tubular epithelial cells in donor kidney biopsies predicts early renal allograft function. J Am Soc Nephrol 10:2006–2013
Wagner MC, Rhodes G, Wang E, Pruthi V, Arif E, Saleem MA, Wean SE, Garg P, Verma R, Holzman LB, Gattone V, Molitoris BA, Nihalani D (2008) Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1. J Biol Chem 283:35579–35589
Snoeijs MGJ, van Heurn LWE, Buurman WA (2010) Biological modulation of renal ischemia-reperfusion injury. Curr Opin Organ Transplant 15:190–199
Li L, Okusa MD (2010) Macrophages, dendritic cells, and kidney ischemia–reperfusion injury. Semin Nephrol 30:268–277
Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD (2007) Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia–reperfusion injury. Kidney Int 71:619–628
De Vries B, Walter SJ, Peutz-Kootstra CJ, Wolfs TGAM, van Heurn LWE, Buurman WA (2004) The mannose-binding lectin-pathway is involved in complement activation in the course of renal ischemia–reperfusion injury. Am J Pathol 165:1677–1688
Van der Pol P, Schlagwein N, van Gijlswijk DJ, Berger SP, Roos A, Bajema IM, de Boer HC, de Fijter JW, Stahl GL, Daha MR, van Kooten C (2012) Mannan-binding lectin mediates renal ischemia/reperfusion injury independent of complement activation. Am J Transplant 12:877–887
Anders HJ (2013) Lech M (2014) NOD-like and Toll-like receptors or inflammasomes contribute to kidney disease in a canonical and a non-canonical manner. Kidney Int 84:225–228
Valles PG, Lorenzo AG, Bocanegra V, Valles R (2014) Acute kidney injury: what part do toll-like receptors play? Int J Nephrol Renov Dis 7:241–251
Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, Han SW, Kim J, Yang CW (2005) Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation 79:1370–1377
Günaydin B, Cakici I, Soncul H, Kalaycioglu S, Cevik C, Sancak B, Kanzik I, Karadenizli Y (2000) Does remote organ ischaemia trigger cardiac preconditioning during coronary artery surgery? Pharmacol Res 41:493–496
Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ, Yellon DM (2007) Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 370:575–579
Venugopal V, Hausenloy DJ, Ludman A, Di Salvo C, Kolvekar S, Yap J, Lawrence D, Bognolo J, Yellon DM (2009) Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart 95:1567–1571
Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, Price V, Tsagakis K, Neuhäuser M, Peters J, Jakob H, Heusch G (2013) Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet 382:597–604
Iliodromitis EK, Kyrzopoulos S, Paraskevaidis IA, Kolocassides KG, Adamopoulos S, Karavolias G, Kremastinos DT (2006) Increased C reactive protein and cardiac enzyme levels after coronary stent implantation. Is there protection by remote ischaemic preconditioning? Heart 92:1821–1826
Hausenloy DJ, Candilio L, Laing C, Kunst G, Pepper J, Kolvekar S, Evans R, Robertson S, Knight R, Ariti C, Clayton T, Yellon DM, Trial Investigators ERICCA (2012) Effect of remote ischemic preconditioning on clinical outcomes in patients undergoing coronary artery bypass graft surgery (ERICCA): rationale and study design of a multi-centre randomized double-blinded controlled clinical trial. Clin Res Cardiol 101:339–348
Wever KE, Menting TP, Rovers M, van der Vliet JA, Rongen GA, Masereeuw R, Ritskes-Hoitinga M, Hooijmans CR, Warlé M (2012) Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS One 7:e32296
Byrne CJ, McCafferty K, Kieswich J, Harwood S, Andrikopoulos P, Raftery M, Thiemermann C, Yaqoob MM (2012) Ischemic conditioning protects the uremic heart in a rodent model of myocardial infarction. Circulation 125:1256–1265
Alreja G, Bugano D, Lotfi A (2012) Effect of remote ischemic preconditioning on myocardial and renal injury: meta-analysis of randomized controlled trials. J Invasive Cardiol 24:42–48
Er F, Nia AM, Dopp H, Hellmich M, Dahlem KM, Caglayan E, Kubacki T, Benzing T, Erdmann E, Burst V, Gassanov N (2012) Ischemic preconditioning for prevention of contrast medium-induced nephropathy: randomized pilot RenPro trial (Renal Protection Trial). Circulation 126:296–303
Wright RS, Reeder GS, Herzog CA, Albright RC, Williams BA, Dvorak DL, Miller WL, Murphy JG, Kopecky SL, Jaffe AS (2002) Acute myocardial infarction and renal dysfunction: a high-risk combination. Ann Intern Med 37:563–570
Bell RM, Rear R, Cunningham J, Dawnay A, Yellon DM (2014) Effect of remote ischaemic conditioning on contrast-induced nephropathy in patients undergoing elective coronary angiography (ERICCIN): rationale and study design of a randomised single-centre, double-blind placebo-controlled trial. Clin Res Cardiol 103:203–209
Gurusamy KS, Kumar Y, Sharma D, Davidson BR (1996) Ischaemic preconditioning for liver transplantation. Cochrane Database Syst Rev 23:CD006315
Chen Y, Zheng H, Wang X, Zhou Z, Luo A, Tian Y (2013) Remote ischemic preconditioning fails to improve early renal function of patients undergoing living-donor renal transplantation. Transplant J 95:e4–e6
Wu J, Feng X, Huang H, Shou Z, Zhang X, Wang R, Chen Y, Chen J (2014) Remote ischemic conditioning enhanced the early recovery of renal function in recipients after kidney transplantation: a randomized controlled trial. J Surg Res 188:303–308
Ali ZA, Callaghan CJ, Lim E, Ali AA, Reza Nouraei SA, Akthar AM, Boyle JR, Varty K, Kharbanda RK, Dutka DP, Gaunt ME (2007) Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation 116:I98–105
Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM, O'Sullivan M, Dutka DP (2009) Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation 119:820–827
Davies WR, Brown AJ, Watson W, McCormick LM, West NEJ, Dutka DP, Hoole SP (2013) Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv 6:246–251
Walsh SR, Boyle JR, Tang TY, Sadat U, Cooper DG, Lapsley M, Norden AG, Varty K, Hayes PD, Gaunt ME (2009) Remote ischemic preconditioning for renal and cardiac protection during endovascular aneurysm repair: a randomized controlled trial. J Endovasc Ther 16:680–689
Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D (2010) Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis 56:1043–1049
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Network AKI (2007) Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11:R31
Rahman IA, Mascaro JG, Steeds RP, Frenneaux MP, Nightingale P, Gosling P, Townsend P, Townend JN, Green D, Bonser RS (2010) Remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment? Circulation 122:S53–59
Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, Jakob H, Heusch G (2010) Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol 105:657–664
Walsh SR, Sadat U, Boyle JR, Tang TY, Lapsley M, Norden AG, Gaunt ME (2010) Remote ischemic preconditioning for renal protection during elective open infrarenal abdominal aortic aneurysm repair: randomized controlled trial. Vasc Endovascular Surg 44:334–340
Zimmerman RF, Ezeanuna PU, Kane JC, Cleland CD, Kempananjappa TJ, Lucas FL, Kramer RS (2011) Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery. Kidney Int 80:861–867
Pedersen KR, Ravn HB, Povlsen JV, Schmidt MR, Erlandsen EJ, Hjortdal VE (2012) Failure of remote ischemic preconditioning to reduce the risk of postoperative acute kidney injury in children undergoing operation for complex congenital heart disease: A randomized single-center study. J Thorac Cardiovasc Surg 143:576–583
Choi YS, Shim JK, Chan Kim J, Kang KS, Seo YH, Ahn KR, Kwak YL (2011) Effect of remote ischemic preconditioning on renal dysfunction after complex valvular heart surgery: A randomized controlled trial. J Thorac Cardiovasc Surg 142:148–154
Hong DM, Jeon Y, Lee CS, Kim HJ, Lee JM, Bahk JH, Kim KB, Hwang HY (2012) Effects of remote ischemic preconditioning with postconditioning in patients undergoing off-pump coronary artery bypass surgery. Circ J 76:884–890
Kim JC, Shim JK, Lee S, Yoo YC, Yang SY, Kwak YL (2012) Effect of combined remote ischemic preconditioning and postconditioning on pulmonary function in valvular heart surgery. Chest 142:467–475
Lee JH, Park YH, Byon HJ, Kim HS, Kim CS, Kim JT (2012) Effect of remote ischaemic preconditioning on ischaemic-reperfusion injury in pulmonary hypertensive infants receiving ventricular septal defect repair. Br J Anaesth 108:223–228
Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, Peters J (2012) Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—a clinical trial. Acta Anaesthesiol Scand 56:30–38
Young PJ, Dalley P, Garden A, Horrocks C, Flamme AL, Mahon B, Miller J, Pilcher J, Weatherall M, Williams J, Young W, Beasley R (2012) A pilot study investigating the effects of remote ischemic preconditioning in high-risk cardiac surgery using a randomised controlled double-blind protocol. Basic Res Cardiol 107:1–10
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P (2004) Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212
Huang J, Chen Y, Dong B, Kong W, Zhang J, Xue W, Liu D, Huang Y (2013) Effect of remote ischaemic preconditioning on renal protection in patients undergoing laparoscopic partial nephrectomy: a “blinded” randomised controlled trial. BJU Int 112:74–80
Igarashi G, Iino K, Watanabe H, Ito H (2013) Remote ischemic pre-conditioning alleviates contrast-induced acute kidney injury in patients with moderate chronic kidney disease. Circ J 77:3037–3044
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Veighey, K., MacAllister, R. Clinical applications of remote ischaemic preconditioning in native and transplant acute kidney injury. Pediatr Nephrol 30, 1749–1759 (2015). https://doi.org/10.1007/s00467-014-2965-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-014-2965-6