Abstract
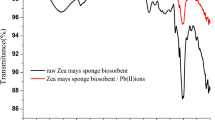
One type of biosorbents, brewer fermentation industry waste yeast, was developed to adsorb the Ag (I) in aqueous solution. The result of FTIR analysis of waste yeast indicated that the ion exchange, chelating and reduction were the main binding mechanisms between the silver ions and the binding sites on the surface of the biomass. Furthermore, TEM, XRD and XPS results suggested that Ag0 nanoparticles were deposited on the surface of yeast. The kinetic experiments revealed that sorption equilibrium could reach within 60 min, and the removal efficiency of Ag (I) could be still over 93 % when the initial concentration of Ag (I) was below 100 mg/L. Thermodynamic parameters of the adsorption process (ΔG, ΔH and ΔS) identified that the adsorption was a spontaneous and exothermic process. The waste yeast, playing a significant role in the adsorption of the silver ions, is useful to fast adsorb Ag (I) from low concentration.








Similar content being viewed by others
References
Wang Y, Ma X, Li Y, Li X, Yang L, Ji L, He Y (2012) Preparation of a novel chelating resin containing amidoxime–guanidine group and its recovery properties for silver ions in aqueous solution. Chem Eng J 209:394–400. doi:10.1016/j.cej.2012.07.143
Sarı A, Tüzen M (2013) Adsorption of silver from aqueous solution onto raw vermiculite and manganese oxide-modified vermiculite. Microporous Mesoporous Mater 170:155–163. doi:10.1016/j.micromeso.2012.12.004
Das N (2010) Recovery of precious metals through biosorption—A review. Hydrometallurgy 103(1–4):180–189. doi:10.1016/j.hydromet.2010.03.016
Sun C, Li C, Qu R, Zhang Y, Bingdong Z, Kuang Y (2014) Syntheses of diethylenetriamine-bridged polysilsesquioxanes and their structure–adsorption properties for Hg(II) and Ag(I). Chem Eng J 240:369–378. doi:10.1016/j.cej.2013.11.092
Huo H, Su H, Tan T (2009) Adsorption of Ag + by a surface molecular-imprinted biosorbent. Chem Eng J 150(1):139–144. doi:10.1016/j.cej.2008.12.014
Wang Y, Gao H, Sun J, Li J, Su Y, Ji Y, Gong C (2011) Selective reinforced competitive biosorption of Ag (I) and Cu (II) on Magnetospirillum gryphiswaldense. Desalination 270(1–3):258–263. doi:10.1016/j.desal.2010.11.053
Huang L, Sun Y, Yang T, Li L (2011) Adsorption behavior of Ni (II) on lotus stalks derived active carbon by phosphoric acid activation. Desalination 268(1–3):12–19. doi:10.1016/j.desal.2010.09.044
Atia AA (2005) Adsorption of silver(I) and gold(III) on resins derived from bisthiourea and application to retrieval of silver ions from processed photo films. Hydrometallurgy 80(1–2):98–106. doi:10.1016/j.hydromet.2005.07.004
Donia AM, Atia AA, Elwakeel KZ (2007) Recovery of gold(III) and silver(I) on a chemically modified chitosan with magnetic properties. Hydrometallurgy 87(3–4):197–206. doi:10.1016/j.hydromet.2007.03.007
Lin Z, Zhou C, Wu J, Zhou J, Wang L (2005) A further insight into the mechanism of Ag + biosorption by Lactobacillus sp. strain A09. Spectrochim Acta A Mol Biomol Spectrosc 61(6):1195–1200. doi:10.1016/j.saa.2004.06.041
Tian L, Wang H, Zhang Y, Sun X, Yuang L (2007) Comprehensive utilization of beer waste in the brewage process. Agr Prod Resour 05:36–42
Singleton I, Simmons P (1996) Factors affecting silver biosorption by an industrial strain of Saccharomyces cerevisiae. J Chem Technol Biotechnol 65(1):21–28
Simmons P, Singleton I (1996) A method to increase silver biosorption by an industrial strain of Saccharomyces cerevisiae. Appl Microbiol Biotechnol 45(1–2):278–285
Yurtsever M, Şengl A (2012) Adsorption and desorption behavior of silver ions onto valonia tannin resin. Trans Nonferrous Met Soc China 22(11):2846–2854. doi:10.1016/s1003-6326(11)61541-0
Pehlivan E, Altun T, Parlayici S (2012) Modified barley straw as a potential biosorbent for removal of copper ions from aqueous solution. Food Chem 135(4):2229–2234. doi:10.1016/j.foodchem.2012.07.017
Puigdomenech I (2002) MEDUSA (make equilibrium diagrams using sophisticated algorithms), vol. 3.1
Cai J, Cui L, Wang Y, Liu C (2009) Effect of functional groups on sludge for biosorption of reactive dyes. J Environ Sci 21(4):534–538. doi:10.1016/s1001-0742(08)62304-9
Abd El-Ghaffar MA, Abdel-Wahab ZH, Elwakeel KZ (2009) Extraction and separation studies of silver(I) and copper(II) from their aqueous solution using chemically modified melamine resins. Hydrometallurgy 96(1–2):27–34. doi:10.1016/j.hydromet.2008.07.008
Jia Y, Demopoulos GP (2003) Adsorption of silver onto activated carbon from acidic media: nitrate and sulfate media. Ind Eng Chem Res 42(1):72–79
Adani KG, Barley RW, Pascoe RD (2005) Silver recovery from synthetic photographic and medical X-ray process effluents using activated carbon. Miner Eng 18(13–14):1269–1276. doi:10.1016/j.mineng.2005.05.021
Atia AA (2005) Adsorption of silver (I) and gold (III) on resins derived from bisthiourea and application to retrieval of silver ions from processed photo films. Hydrometallurgy 80(1):98–106
Donia AM, Atia AA, Elwakeel KZ (2007) Recovery of gold (III) and silver (I) on a chemically modified chitosan with magnetic properties. Hydrometallurgy 87(3):197–206
Wang L, Xing R, Liu S, Yu H, Qin Y, Li K, Feng J, Li R, Li P (2010) Recovery of silver (I) using a thiourea-modified chitosan resin. J Hazard Mater 180(1–3):577–582. doi:10.1016/j.jhazmat.2010.04.072
Safarik I, Rego LFT, Borovska M, Mosiniewicz-Szablewska E, Weyda F, Safarikova M (2007) New magnetically responsive yeast-based biosorbent for the efficient removal of water-soluble dyes. Enzym Microb Technol 40(6):1551–1556. doi:10.1016/j.enzmictec.2006.10.034
Cui L, Wu G, Deng K, Won SW, Yun Y (2006) Application of RR 4 biosorption models. Fresen Environ Bull 15(11):1442–1446
Cui L, Liu C, Wu G (2008) Performance and mechanism of methylene blue biosorption on orange peel. Environ Technol 29(9):1021–1030
Song X, Gunawan P, Jiang R, Leong SSJ, Wang K, Xu R (2011) Surface activated carbon nanospheres for fast adsorption of silver ions from aqueous solutions. J Hazard Mater 194:162–168. doi:10.1016/j.jhazmat.2011.07.076
Das D, Das N, Mathew L (2010) Kinetics, equilibrium and thermodynamic studies on biosorption of Ag(I) from aqueous solution by macrofungus Pleurotus platypus. J Hazard Mater 184(1–3):765–774. doi:10.1016/j.jhazmat.2010.08.105
Li Y, Duan X, Qian Y, Yang L, Liao H (1999) Nanocrystalline silver particles: synthesis, agglomeration, and sputtering induced by electron beam. J Colloid Interface Sci 209(2):347–349
Song JY, Kim BS (2008) Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng 32(1):79–84. doi:10.1007/s00449-008-0224-6
Deng S, Ting YP (2005) Characterization of PEI-modified biomass and biosorption of Cu(II), Pb(II) and Ni(II). Water Res 39(10):2167–2177. doi:10.1016/j.watres.2005.03.033
Won SW, Wu G, Ma H, Liu Q, Yan Y, Cui L, Liu C, Yun YS (2006) Adsorption performance and mechanism in binding of Reactive Red 4 by coke waste. J Hazard Mater 138(2):370–377. doi:10.1016/j.jhazmat.2006.05.060
Cui L, Wu G, Jeong T-s (2010) Adsorption performance of nickel and cadmium ions onto brewer’s yeast. Can J Chem Eng 88(1):109–115. doi:10.1002/cjce.20241
Li XG, Feng H, Huang MR (2010) Redox sorption and recovery of silver ions as silver nanocrystals on poly(aniline-co-5-sulfo-2-anisidine) nanosorbents. Chemistry 16(33):10113–10123. doi:10.1002/chem.201000506
Luo C, Zhang Y, Zeng X, Zeng Y, Wang Y (2005) The role of poly(ethylene glycol) in the formation of silver nanoparticles. J Colloid Interface Sci 288(2):444–448. doi:10.1016/j.jcis.2005.03.005
Won SW, Mao J, Kwak IS, Sathishkumar M, Yun YS (2010) Platinum recovery from ICP wastewater by a combined method of biosorption and incineration. Bioresour Technol 101(4):1135–1140. doi:10.1016/j.biortech.2009.09.056
Acknowledgments
This work was sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, Y., Wang, D., Xie, H. et al. Adsorption of Ag (I) from aqueous solution by waste yeast: kinetic, equilibrium and mechanism studies. Bioprocess Biosyst Eng 38, 69–77 (2015). https://doi.org/10.1007/s00449-014-1244-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1244-z