Abstract
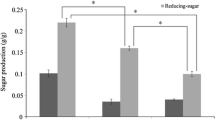
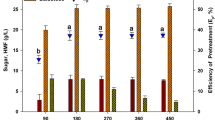
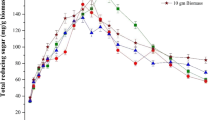
Ethanol was produced using the simultaneous saccharification and fermentation (SSF) method with macroalgae polysaccharide from the seaweed Saccharina japonica (Sea tangle, Dasima) as biomass. The seaweed was dried by hot air, ground with a hammer mill and filtered with a 200-mesh sieve prior to pretreatment. Saccharification was carried out by thermal acid hydrolysis with H2SO4 and the industrial enzyme, Termamyl 120 L. To increase the yield of saccharification, isolated marine bacteria were used; the optimal saccharification conditions were 10% (w/v) seaweed slurry, 40 mM H2SO4 and 1 g dcw/L isolated Bacillus sp. JS-1. Using this saccharification procedure, the reducing sugar concentration and viscosity were 45.6 ± 5.0 g/L and 24.9 cp, respectively, and the total yield of the saccharification with optimal conditions and S. japonica was 69.1%. Simultaneous saccharification and fermentation was carried out for ethanol production. The highest ethanol concentration, 7.7 g/L (9.8 ml/L) with a theoretical yield of 33.3%, was obtained by SSF with 0.39 g dcw/L Bacillus sp. JS-1 and 0.45 g dcw/L of the yeast, Pichia angophorae KCTC 17574.







Similar content being viewed by others
References
Cazetta M, Celligoi M, Buzato J, Scarmino I (2007) Fermentation of molasses by Zymomonas mobilis: effects of temperature and sugar concentration on ethanol production. Bioresour Technol 98(15):2824–2828
Davis L, Jeon Y, Svenson C, Rogers P, Pearce J, Peiris P (2005) Evaluation of wheat stillage for ethanol production by recombinant Zymomonas mobilis. Biomass Bioenergy 29(1):49–59
Berndes G, Hoogwijk M, van den Broek R (2003) The contribution of biomass in the future global energy supply: a review of 17 studies. Biomass Bioenergy 25(1):1–28
Karakashev D, Thomsen A, Angelidaki I (2007) Anaerobic biotechnological approaches for production of liquid energy carriers from biomass. Biotechnol Lett 29(7):1005–1012
Bothast R, Schlicher M (2005) Biotechnological processes for conversion of corn into ethanol. J Appl Microbiol Biotechnol 67(1):19–25
Lu X, Zhang Y, Angelidaki I (2009) Optimization of H2SO4-catalyzed hydrothermal pretreatment of rapeseed straw for bioconversion to ethanol: focusing on pretreatment at high solids content. Bioresour Technol 100(12):3048–3053
Adams J, Gallagher J, Donnison I (2009) Fermentation study on Saccharina latissima for bioethanol production considering variable pre-treatments. J Appl Phycol 21(5):569–574
McHugh DJ (2003) A guide to the seaweed industry. FAO Fish Tech Pap
Horn S, Aasen I, Østgaard K (2000) Ethanol production from seaweed extract. J Ind Microbiol 25(5):249–254
Horn S, Aasen I, Østgaard K (2000) Production of ethanol from mannitol by Zymobacter palmae. J Ind Microbiol Biotechnol 24(1):51–57
Tang JC, Taniguchi H, Chu H, Zhou Q, Nagata S (2009) Isolation and characterization of alginate-degrading bacteria for disposal of seaweed wastes. Lett Appl Microbiol 48(1):38–43
Choi DB, Sim HS, Piao YL, Ying W, Cho H (2009) Sugar production from raw seaweed using the enzyme method. J Ind Eng Chem 15:12–15
Kloareg B, Quatrano R (1988) Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr Mar Biol Annu Rev 26:259–315
Myklestad S (1978) Beta-1, 3-glucans in diatoms and brown seaweeds, Handbook of phycological methods. Cambridge University Press, Cambridge, UK
Kuhad R, Gupta R, Khasa Y, Singh A (2010) Bioethanol production from Lantana camara (red sage): Pretreatment, saccharification and fermentation. Bioresour Technol 101:8348–8354
Tomas-Pejo E, Garcia-Aparicio M, Negro M, Oliva J, Ballesteros M (2009) Effect of different cellulase dosages on cell viability and ethanol production by Kluyveromyces marxianus in SSF processes. Bioresour Technol 100(2):890–895
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83(1):1–11
Na JB, Kim JS (2008) The optimum condition of SSF to ethanol production from starch biomass. Korean Chem Eng Res 46(5):858–862
Stenberg K, Bollok M, Reczey K, Galbe M, Zacchi G (2000) Effect of substrate and cellulase concentration on simultaneous saccharification and fermentation of steam-pretreated softwood for ethanol production. Biotechnol Bioeng 68(2):204–210
Alfani F, Gallifuoco A, Saporosi A, Spera A, Cantarella M (2000) Comparison of SHF and SSF processes for the bioconversion of steam-exploded wheat straw. J Ind Microbiol Biotechnol 25(4):184–192
Wingren A, Galbe M, Zacchi G (2003) Techno-economic evaluation of producing ethanol from softwood: comparison of SSF and SHF and identification of bottlenecks. Biotechnol Prog 19(4):1109–1117
Wei G, Gao W, Jin I, Yoo S, Lee J, Chung C, Lee J (2009) Pretreatment and saccharification of rice hulls for the production of fermentable sugars. Biotechnol Bioprocess Eng 14(6):828–834
Tomas-Pejo E, Oliva J, Gonzalez A, Ballesteros I, Ballesteros M (2009) Bioethanol production from wheat straw by the thermotolerant yeast Kluyveromyces marxianus CETC 10875 in a simultaneous saccharification and fermentation fed-batch process. Fuel 88:2142–2147
Lee S, Kim J, Cho H, Joo H, Lee J (2009) Production of bio-ethanol from brown algae by physicochemical hydrolysis. J Korean Ind Eng Chem 20(5):517–521
Lee S, Lee J (2010) Influence of acid and salt content on the ethanol production from Laminaria japonica. Appl Chem Eng 21(2):154–161
Dubois M, Gilles K, Hamilton J, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Biochem 28(3):350–356
Zacchi G, Axelsson A (1989) Economic evaluation of preconcentration in production of ethanol from dilute sugar solutions. Biotechnol Bioeng 34(2):223–233
Li H, Kim N, Jiang M, Kang J, Chang H (2009) Simultaneous saccharification and fermentation of lignocellulosic residues pretreated with phosphoric acid-acetone for bioethanol production. Bioresour Technol 100(13):3245–3251
Horn S (2000) Bioenergy from brown seaweeds. Norwegian University of Science and Technology NTNU, Trondheim
Horn S, Østgaard K (2001) Alginate lyase activity and acidogenesis during fermentation of Laminaria hyperborea. J Appl Phycol 13(2):143–152
Acknowledgments
This research was supported by a grant from the Development of Marine-Bioenergy Program Funded by the Ministry of Land, Transport and Maritime Affairs of the Korean Government. Ji-Suk Jang and YuKyeong Cho were financially supported by the Brain Busan 21 HRD Project of the Metropolitan City of Busan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jang, JS., Cho, Y., Jeong, GT. et al. Optimization of saccharification and ethanol production by simultaneous saccharification and fermentation (SSF) from seaweed, Saccharina japonica . Bioprocess Biosyst Eng 35, 11–18 (2012). https://doi.org/10.1007/s00449-011-0611-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0611-2