Abstract
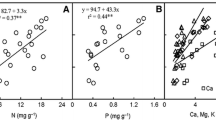
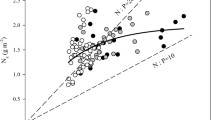
Photosynthetic capacity of tree leaves is typically positively related to nutrient content and little affected by changes in growth temperature. These relationships are, however, often poorly supported for tropical trees, for which interspecific differences may be more strongly controlled by within-leaf nutrient allocation than by absolute leaf nutrient content, and little is known regarding photosynthetic acclimation to temperature. To explore the influence of leaf nutrient status, successional strategy and growth temperature on the photosynthetic capacity of tropical trees, we collected data on photosynthetic, chemical and morphological leaf traits of ten tree species in Rwanda. Seven species were studied in a forest plantation at mid-altitude (~1,700 m), whereas six species were studied in a cooler montane rainforest at higher altitude (~2,500 m). Three species were common to both sites, and, in the montane rainforest, three pioneer species and three climax species were investigated. Across species, interspecific variation in photosynthetic capacity was not related to leaf nutrient content. Instead, this variation was related to differences in within-leaf nitrogen allocation, with a tradeoff between investments into compounds related to photosynthetic capacity (higher in pioneer species) versus light-harvesting compounds (higher in climax species). Photosynthetic capacity was significantly lower at the warmer site at 1,700 m altitude. We conclude that (1) within-leaf nutrient allocation is more important than leaf nutrient content per se in controlling interspecific variation in photosynthetic capacity among tree species in tropical Rwanda, and that (2) tropical montane rainforest species exhibit decreased photosynthetic capacity when grown in a warmer environment.





Similar content being viewed by others
References
Ames BN (1966) Assay of inorganic phosphate, total phosphate and phosphatases. Method Enzymol 8:115–118
Beer C, Reichstein M, Tomelleri E, Ciais P, Jung M, Carvalhais N, Rodenbeck C, Arain MA, Baldocchi D, Bonan GB, Bondeau A, Cescatti A, Lasslop G, Lindroth A, Lomas M, Luyssaert S, Margolis H, Oleson KW, Roupsard O, Veenendaal E, Viovy N, Williams C, Woodward FI, Papale D (2010) Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science 329:834–838
Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ 24:253–259
Bernacchi CJ, Pimentel C, Long SP (2003) In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant Cell Environ 26:1419–1430
Bloesch U, Troupin G, Derungs N (2009) Les plantes ligneuses du Rwanda Flore, ecologie et usages. Shaker, Aachen
Booth BBB, Jones CD, Collins M, Totterdell IJ, Cox PM, Sitch S, Huntingford C, Betts RA, Harris GR, Lloyd J (2012) High sensitivity of future global warming to land carbon cycle processes. Environ Res Lett 7:024002
Bruijnzeel LA, Scatena FN, Hamilton L (2010) Tropical montane cloud forests: science for conservation and management. Cambridge University Press, New York
Carswell FE, Meir P, Wandelli EV, Bonates LCM, Kruijt B, Barbosa EM, Nobre AD, Grace J, Jarvis PG (2000) Photosynthetic capacity in a central Amazonian rain forest. Tree Physiol 20:179–186
Chao N, Mulindahabi F, Easton J, Plumptre AJ, Seimon A, Martin A, Fimbel R (2011) Long term changes in a montane forest in a region of high population density. In: Plumptre AJ (ed) The ecological impact of Long-term Changes in Africa’s rift valley. Nova Science, New York, pp 167–202
Clark DA (2004) Sources or sinks? The response of tropical forests to current and future climate and atmospheric composition. Philos Trans R Soc Lond B 359:477–491
Coste S, Roggy JC, Imbert P, Born C, Bonal D, Dreyer E (2005) Leaf photosynthetic traits of 14 tropical rain forest species in relation to leaf nitrogen concentration and shade tolerance. Tree Physiol 25:1127–1137
Coste S, Baraloto C, Leroy C, Marcon E, Renaud A, Richardsson AD, Roggy J-C, Schimann H, Uddling J, Hérault B (2010) Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: a calibration test with thirteen tree species of tropical rainforest in French Guiana. Ann For Sci 67:607
Davidson EA, Reis de Carvalho CJ, Figueira AM, Ishida FY, Ometto JPHB, Nardoto GB, Saba RT, Hayashi SN, Leal EC, Vieira ICG, Martinelli LA (2007) Recuperation of nitrogen cycling in Amazonian forests following agricultural abandonment. Nature 447:995–998
Domingues TF, Berry JA, Martinelli LA, Ometto JPHB, Ehleringer JR (2005) Parameterization of canopy structure and leaf-level gas exchange for an astern Amazonian tropical rain forest (Tapajós National Forest, Pará, Brazil). Earth Interact 9:1–23
Domingues TF, Meir P, Feldpausch TR, Saiz G, Veenendaal EM, Schrodt F, Bird M, Djagbletey G, Hien F, Compaore H, Diallo A, Grace J, Llyod J (2010) Co-limitation of photosynthetic capacity by nitrogen and phosphorus in West Africa woodlands. Plant Cell Environ 33:959–980
Doughty CE, Goulden ML (2008) Are tropical forests near a high temperature threshold? J Geophys Res Biogeosci 113:G00B07
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
FAO (2001) Global forest resources assessment 2000—main report. FAO Forestry Paper 140. United Nations Food and Agricultural Organisation, Rome
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Fischer E, Killmann D (2008) Illustrated field guide to the plants of Nyungwe National Park Rwanda. Koblenz Geographical Colloquia Series Biogeographical Monographs 1. Koblenz, Koenigstein
Fisher JB, Malhi Y, Torres IC, Metcalfe DB, van de Weg MJ, Meir P, Silva-Espejo JE, Huasco WH (2012) Nutrient limitation in rainforests and cloud forests along a 3,000 m elevation gradient in the Peruvian Andes. Oecologia 172:889–902
Friedlingstein P, Cox P, Betts R, Bopp L, Von Bloh W, Brovkin V, Cadule P, Doney S, Eby M, Fung I, Bala G, John J, Jones C, Joos F, Kato T, Kawamiya M, Knorr W, Lindsay K, Matthews HD, Raddatz T, Rayner P, Reick C, Roeckner E, Schnitzler KG, Schnur R, Strassmann K, Weaver AJ, Yoshikawa C, Zeng N (2006) Climate carbon cycle feedback analysis, results from the C4MIP model intercomparison. J Climate 19:3337–3353
Hikosaka K (1997) Modelling optimal temperature acclimation of the photosynthetic apparatus in C3 plants with respect to nitrogen use. Ann Bot 80:721–730
Houter NC, Pons TL (2012) Ontogenetic changes in leaf traits of tropical rainforest trees differing in juvenile light requirement. Oecologia 169:33–45
IPCC (2013) Climate change, the physical science basis. In: Stocker T (ed) Summary for policymakers. Cambridge University Press, Cambridge
Kattge J, Knorr W (2007) Temperature acclimation in a biochemical model of photosynthesis: a reanalysis of data from 36 species. Plant Cell Environ 30:1176–1190
Kattge J, Knorr W, Raddatz T, Wirth C (2009) Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global-scale terrestrial biosphere models. Glob Change Biol 15(4):976–991
Lewis SL (2006) Tropical forests and the changing of earth system. Philos Trans R Soc Lond B 361:195–210
Medlyn BE, Dreyer E, Ellsworth D, Forstreuter M, Harley PC, Kirschbaum MUF, Le Roux X, Montpied P, Strassemeyer J, Walcroft A, Wang K, Loustau D (2002) Temperature response of parameters of biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ 25:1167–1179
Meir P, Levy PE, Grace J, Jarvis PG (2007) Photosynthetic parameters from two contrasting woody vegetation types in West Africa. Plant Ecol 192:277–287
Mercado LM, Patiño S, Domingues TF, Fyllas NM, Weedon GP, Sitch S, Quesada CA, Phillips OL, Aragão LEOC, Malhi Y, Dolman AJ, Restrepo-Coupe N, Saleska SR, Baker TR, Almeida S, Higuchi N, Lloyd J (2011) Variations in Amazon forest productivity correlated with foliar nutrients and modelled rates of photosynthetic carbon supply. Philos Trans R Soc Lond B 366:3316–3329
Nduwayezu JB, Ruffo CK, Minani V, Munyaneza E, Nshutiyayesu S, Institute of Scientific and Technological Research (IRST) (2009) Know some useful trees and shrubs for agricultural and pastoral communities of Rwanda. Palotti, Kigali
Nsabimana D (2009) Carbon stock and fluxes in Nyungwe forest and Ruhande Arboretum in Rwanda. PhD thesis, University of Gothenburg, Gothenburg
Nsabimana D, Klemedtson L, Kaplin BA, Wallin G (2009) Soil CO2 flux in six monospecific forest plantations in Southern Rwanda. Soil Biol Biochem 41:396–402
Orwa C, Mutua A, Kindt R, Jamnadass R, Anthony S (2009) Agroforestree database: a tree reference and selection guide version 4.0. World Agroforestry Centre, Nairobi
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG, Ciais P, Jackson RB, Pacala SW, McGuire AD, Piao S, Rautiainen A, Sitch S, Hayes D (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–993
Plumptre AJ, Masozera M, Fashing PJ, McNeilage A, Ewango C, Kaplin BA, Liengola I (2002) Biodiversity surveys of the Nyungwe forest reserve in SW Rwanda. WCS working papers no. 18
Raaimakers D, Boot RGA, Dijkstra P, Pot S, Pons T (1995) Photosynthetic rates in relation to leaf phosphorous content in pioneer versus climax tropical rainforest trees. Oecologia 102:120–125
Rozendaal DMA, Hurtado VH, Poorter L (2006) Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Funct Ecol 20:207–216
Sellers PJ, Dickinson RE, Randall DA, Betts AK, Hall FG, Berry JA, Collatz GJ, Denning AS, Mooney HA, Nobre CA, Sato N, Field CB, Henderson-Sellers A (1997) Modeling the exchanges of energy, water, and carbon between continents and the atmosphere. Science 275:502–509
Uddling J, Gelang-Alfredsson J, Piikki K, Pleijel H (2007) Evaluating the relationship between SPAD-502 chlorophyll meter readings and leaf chlorophyll concentration. Photosynth Res 91:37–46
Valderrama GC (1981) The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Mar Chem 10:109–112
Valladares F, Niinemets Ü (2008) Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Evol Syst 39:237–257
van Bodegom PM, Douma JC, Witte JPM, Ordonez JC, Bartholomeus RP, Aerts R (2012) Going beyond limitations of plant functional types when predicting global ecosystem-atmosphere fluxes: exploring the merits of traits-based approaches. Glob Ecol Biogeogr 21:625–636
van de Weg MJ, Meir P, Grace J, Ramos GD (2012) Photosynthetic parameters, dark respiration and leaf traits in the canopy of a peruvian tropical montane cloud forest. Oecologia 168:23–34
Vårhammar A, Wallin G, McLean CM, Dusenge ME, Medlyn BE, Hasper TB, Nsabimana D, Uddling J (2015) Photosynthetic temperature responses of tree species in Rwanda: evidence of pronounced negative effects of high temperature in montane rainforest climax species. New Phytol. doi:10.1111/nph.13291
Vitousek PM (1984) Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 65:285–298
von Caemmerer S (2000) Biochemical models of leaf photosynthesis. CSIRO, Collingwood
Way DA, Oren R (2010) Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol 30:669–688
Wullschleger SD (1993) Biochemical limitations to carbon assimilation in C 3 plants a retrospective analysis of the A/C i curves from 109 species. J Exp Bot 44:907–920
Yamori W, Noguchi K, Terashima I (2005) Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant Cell Environ 28:536–547
Acknowledgments
The first author was supported by a scholarship within the Global university initiative at University of Gothenburg, Sweden, and the last author was supported by Helge Ax:son Johnsons Stiftelse and the Strategic Research Area “Biodiversity and Ecosystem Services in a Changing Climate” (BECC; http://www.cec.lu.se/research/becc). We are grateful to the Swedish International Development Cooperation Agency (SIDA) support to infrastructure and to Rwanda Agricultural Board (RAB) Ruhande and Rwanda Development Board (RDB) which authorized data collection in the Ruhande Arboretum and Nyungwe National Park, respectively. We are also grateful for comments on a draft of this manuscript by Angelica Vårhammar.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Gerardo Avalos.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dusenge, M.E., Wallin, G., Gårdesten, J. et al. Photosynthetic capacity of tropical montane tree species in relation to leaf nutrients, successional strategy and growth temperature. Oecologia 177, 1183–1194 (2015). https://doi.org/10.1007/s00442-015-3260-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3260-3