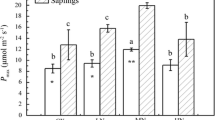
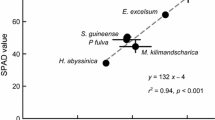
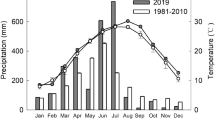
Abstract
Rainforest tree species are often found on poor soils where essential nutrients may be in low concentrations. Here, we determine the effect of nutrients (N, P, Ca, Mg and K) in photosynthetic traits in five rainforest tree species from central Amazonia. Gas exchange parameters were measured with an infrared gas analyzer in four saplings (1–3 m tall) per species using two leaves per plant. Data on gas exchange and leaf nutrient content (N, P, K, Ca and Mg) were collected between August and November, 2010. Specific leaf area (SLA) and leaf thickness (L T) were also determined. Potential photosynthesis per unit mass (A pot-mass), maximum carboxylation velocity of Rubisco (V c-max) and electron transport rate (J max) were responsive to variation in leaf nutrient content per unit mass. On a mass basis, P content was positively correlated with N, Mg, and K content; Mg content was positively correlated with K content. However, no correlation was found between the content of P, Mg, K and Ca, or between Ca, Mg, K and N. SLA and L T were strongly related to A pot-area, V c-max and J max (per unit area). Our study shows in situ evidence on the effect of leaf nutrients on A pot-mass, V c-max and J max (per unit mass) of tree saplings in the central Amazon. The magnitude of changes in photosynthetic capacity of juvenile trees in the forest understory depends not only on N and P use efficiency, but also on the availability of K, Mg and Ca, in decreasing order.



Similar content being viewed by others
Abbreviations
- A pot-area :
-
Potential photosynthesis per unit area
- A pot-mass :
-
Potential photosynthesis per unit mass
- A pot-area/Ca:
-
Calcium use efficiency
- A pot-area/K:
-
Potassium use efficiency
- A pot-area/Mg:
-
Magnesium use efficiency
- A pot-area/N:
-
Nitrogen use efficiency
- A pot-area/P:
-
Phosphorus use efficiency
- Ca:
-
Calcium
- C i :
-
Intercellular CO2 concentration
- J max :
-
Electron transport rate
- K:
-
Potassium
- K c :
-
Michaelis constant of Rubisco for carboxylation
- K o :
-
Michaelis constant of Rubisco for oxygenation
- L T :
-
Fresh leaf thickness
- Mg:
-
Magnesium
- N:
-
Nitrogen
- P:
-
Phosphorous
- PPFD:
-
Photosynthetic photon flux density
- SLA:
-
Specific leaf area
- V cmax :
-
Maximum carboxylation velocity of Rubisco
- Γ*:
-
CO2 compensation point in the absence of mitochondrial respiration in an illuminated leaf
References
Aerts R, Chapin FS III (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Broadley MR et al (2004) Phylogenetic variation in the shoot mineral concentration of angiosperms. J Exp Bot 55:321–336
Brown MS, Bethlenfalvay GJ (1988) The Glycine-Glomus-Rhizobium symbiosis. VII. Photosynthetic nutrient-use efficiency in nodulated, mycorrhizal soybeans. Plant Physiol 86:1292–1297
Cordell S, Goldstein G, Meinzer FC, Vitousek PM (2001) Regulation of leaf life-span and nutrient-use efficiency of Metrosideros polymorpha trees at two extremes of a long chronosequence in Hawaii. Oecologia 127:198–206
Cuevas E, Medina E (1988) Nutrient dynamics within Amazonian forests. II. Fine root-growth, nutrient availability and leaf litter decomposition. Oecologia 76:222–235
Drechsel P, Zech W (1991) Foliar nutrient levels of broad-leaved tropical trees: a tabular review. Plant Soil 131:29–46
Elser JJ et al (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Farquhar GD, Von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Fyllas NM et al (2009) Basin-wide variations in foliar properties of Amazonian forest: phylogeny, soils and climate. Biogeosciences 6:2677–2708
Godde D, Hefer M (1994) Photoinhibition and light-dependent turnover of the D1 reaction-centre polypeptide of photosystem II are enhanced by mineral-stress conditions. Planta 193:290–299
Güsewell SN (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Güsewell S, Koerselman W (2002) Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect Ecol Evol Syst 5:37–61
Hidaka A, Kitayama K (2009) Divergent patterns of photosynthetic phosphorus-use efficiency versus nitrogen-use efficiency of tree leaves along nutrient-availability gradients. J Ecol 97:984–991
Hikosaka K (2004) Interspecific difference in the photosynthesis-nitrogen relationship: patterns, physiological causes, and ecological importance. J Plant Res 117:481–494
Hiremath AJ (2000) Photosynthetic nutrient-use efficiency in three fast-growing tropical trees with differing leaf longevities. Tree Physiol 20:937–944
Houlton BZ, Sigman DM, Hedin LO (2006) Isotopic evidence for large gaseous nitrogen losses from tropical rainforests. Proc Natl Acad Sci USA 103:8745–8750
Jia Y, Gray VM (2004) Influence of phosphorus and nitrogen on photosynthetic parameters and growth in Vicia faba L. Photosynthetica 42:535–542
Kaspari M et al (2008) Multiple nutrients limit litterfall and decomposition in a tropical forest. Ecol Lett 11:35–43
Kitajima K, Mulkey SS, Wright SJ (1997) Decline of photosynthetic capacity with leaf age in relation to leaf longevities for five tropical canopy tree species. Am J Bot 84:702–708
Koerselman W, Meuleman AFM (1996) The vegetation N: p ratio: A new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450
Köppen W (1936) Das geographische System der Klimate. In: Koppen W, Geiger R (eds) Handbuch der Klimatologie. Gebrüder Bornträger, Berlin, pp 1–44
Lin Z-H, Chen L-S, Chen R-B, Zhang F-Z, Jiang H-X, Tang N (2009) CO2 assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport probed by the JIP-test, of tea leaves in response to phosphorus supply. BMC Plant Biol 9:43. doi:10.1186/1471-2229-9-43
Lloyd J, Syvertsen JP, Kriedemann PE, Farquhar GD (1992) Low conductances for CO2 diffusion from stomata to the sites of carboxylation in leaves of woody species. Plant, Cell Environ 12:873–899
Magalhães, N.S. (2010) [Growth and diurnal variation in photosynthesis and stomatal conductance in five Amazonian tree species]. MSc. Dissertation. Botany Graduate Program. Instituto Nacional de Pesquisas da Amazônia, Manaus. [In Portuguese]
Magalhães NS, Marenco RA, Camargo MAB (2014) Do soil fertilization and forest canopy foliage affect the growth and photosynthesis of Amazonian saplings? Sci Agric 71:58–65
Manter DK, Kerrigan J (2004) A/C i curve analysis across a range of woody plant species: influence of regression analysis parameters and mesophyll conductance. J Exp Bot 55:2581–2588
Marenco RA, Nascimento HCS, Magalhães NS (2014) Stomatal conductance in Amazonian tree saplings in response to variations in the physical environment. Photosynthetica 52:493–500
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London, p 889
Medlyn BE et al (1999) Effects of elevated [CO2] on photosynthesis in European forest species: a meta-analysis of model parameters. Plant, Cell Environ 22:1475–1495
Mendes KR, Marenco RA (2010) Leaf traits and gas exchange in saplings of native tree species in the Central Amazon. Sci Agric 67:624–632
Mendes KR, Marenco RA, Magalhães NS (2013) Growth and photosynthetic use efficiency of nitrogen and phosphorus in saplings of Amazonian tree species. Rev Árvore 37:707–716 [In Portuguese]
Oguchi R, Hikosaka K, Hirose T (2005) Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant, Cell Environ 28:916–927
Quesada CA et al (2010) Variations in chemical and physical properties of Amazon forest soils in relation to their genesis. Biogeosciences 7:1515–1541
Raaimakers D, Boot RGA, Dijkstra R, Pot S, Pons T (1995) Photosynthetic rates in relation to leaf phosphorus content in pioneer versus climax tropical rainforest trees. Oecologia 102:120–125
Raich JW, Russell AE, Crews TE, Farrington H, Vitousek PM (1996) Both nitrogen and phosphorus limit plant production on young Hawaiian lava flows. Biogeochemistry 32:1–14
Reich PB et al (1999) Generality of leaf trait relationships: a test across six biomes. Ecology 80:1955–1969
Tilman D, Lehman CL, Thomas KT (1997) Plant diversity and ecosystem productivity: theoretical considerations. Proc Natl Acad Sci USA 94:1857–1861
Townsend AR, Cleveland CC, Houlton BZ, Alden CB, White JWC (2011) Multi-element regulation of the tropical forest carbon cycle. Front Ecol Environ 9:9–17
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13:87–115
Vitousek PM, Sanford RL (1986) Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst 17:137–167
Warren CR (2004) The photosynthetic limitation posed by internal conductance to CO2 movement is increased by nutrient supply. J Exp Bot 55:2313–2321
Warren CR et al (2003) Transfer conductance in second growth Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) canopies. Plant, Cell Environ 26:1215–1227
Weih M, Asplund L, Bergkvist G (2011) Assessment of nutrient use in annual and perennial crops: a functional concept for analyzing nitrogen use efficiency. Plant Soil 339:513–520
Wright IJ et al (2005) Assessing the generality of global leaf trait relationships. New Phytol 166:485–496
Wullschleger SD (1993) Biochemical limitations to carbon assimilation in C3 plants—a retrosprective analysis of the A/Ci curves from 109 species. J Exp Bot 44:907–920
Wykoff DD, Davies JP, Melis A, Grossman AR (1998) The regulation of photosynthetic electron-transport during nutrient deprivation in Chlamydomonas reinhardtii. Plant Physiol 117:129–139
Yu-Long F, Gai-Lan F, Yu-Long Z (2008) Specific leaf area relates to the differences in leaf construction cost, photosynthesis, nitrogen allocation, and use efficiencies between invasive and noninvasive alien congeners. Planta 228:383–390
Acknowledgments
Ministério da Ciência Tecnologia e Inovação (MCTI/INPA), Fundação de Amparo à Pesquisa do Estado do Amazonas, FAPEAM (UA-062.03164.12), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mendes, K.R., Marenco, R.A. Photosynthetic traits of tree species in response to leaf nutrient content in the central Amazon. Theor. Exp. Plant Physiol. 27, 51–59 (2015). https://doi.org/10.1007/s40626-015-0031-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-015-0031-9