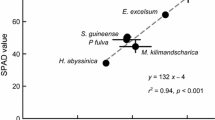
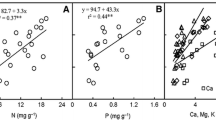
Abstract
Measurements of leaf gas exchange were made in contrasting wooded ecosystems in West Africa. Measurements were made on 10 species: seven from humid rain forest in Cameroon and three from the semi-arid Sahelian zone in Niger. For each species, two models of photosynthesis were fitted: the first based on a rectangular hyperbolic response to photosynthetic photon flux density (Q), and the second the biochemical model of Farquhar et al. (1980). In both communities, the species studied could be divided into those characteristic of early and late successional stages, but photosynthetic parameters were not closely related to successional stage. The data identified significant relationships between V cmax and leaf nutrient (N and P) content when expressed on an area basis. Variation in leaf mass per unit area correlated with canopy exposure and dominated the leaf nutrient signal. Statistical analysis suggested weakly that leaf gas exchange was more limited by P than N at the rain forest site.



Similar content being viewed by others
References
Ambouta K (1984) Contribution a l’edaphologie de la brousse tigree de l’ouest nigerien. These de Docteur Ingenieur, Universite de Nancy I
Anten NPR, Schieving F, Werger MJA (1995) Patterns of light and nitrogen distribution in relation to whole canopy carbon gain in C-3 and C-4 monocotyledonous and dicotyledonous species. Oecologia 101:504–513
Atkin OK, Evans JR, Ball MC et al (2000) Leaf respiration of snow gum in the light and dark interactions between temperature and irradiance. Plant Physiol 122:915–923
Bazzaz FA, Pickett STA (1980) Physiological ecology of tropical succession: a comparative review. Annu Rev Ecol Syst 11:237–310
Beerling DJ, Quick WP (1995) A new technique for estimating rates of carboxylation and electron-transport in leaves of C-3 plants for use in dynamic global vegetation models. Glob Chan Biol 1:289–294
Bernacchi CJ, Singsaas EL, Pimentel C et al (2001). Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Envir 24:253–259
Brooks A, Farquhar GD (1985) Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase oxygenase and the rate of respiration in the light—estimates from gas-exchange measurements on spinach. Planta 165:397–406
Burns AE, Gleadow RM, Woodrow IE (2002) Light alters the allocation of nitrogen to cyanogenic glycosides in Eucalyptus cladocalyx. Oecologia 133:288–294
Carswell FE, Meir P, Wandelli EV et al (2000) Photosynthetic capacity in a central Amazonian rain forest. Tree Physiol 20:179–186
Chazdon RL, Field CB (1987) Determinants of photosynthetic capacity in 6 rain forest species. Oecologia 73:222–230
Chen JL, Reynolds JF, Harley PC et al (1993) Coordination theory of leaf nitrogen distribution in a canopy. Oecologia 93:63–69
Coste S, Roggy J-C, Imbert P et al (2005) Leaf photosynthetic traits of 14 tropical rain forest species in relation to leaf nitrogen concentration and shade tolerance. Tree Physiol 25:1127–1137
Culf AD, Allen SJ, Gash JHC et al (1993) Energy and water budgets of an area of patterned woodland in the Sahel. Agric For Meteorol 66:65–80
De Pury DGG, Farquhar GD (1997) Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant Cell Envir 20:537–557
D’Mello JPF (1995) Toxicity of non-protein amino acids from plants. In: Wallsgrove RM (ed) Amino acids and their derivatives in higher plants. Cambridge University Press, Cambridge
Domingues TF, Berry JA, Martinelli LA et al (2005) Parameterization of canopy structure and leaf-level gas exchange for an eastern Amazonian tropical rain forest (Tapajos National Forest, Para, Brazil). Earth Interact 9:1–23
Ellsworth DS, Reich PB (1996) Photosynthesis and leaf nitrogen in five Amazonian tree species during early secondary succession. Ecology 77:581–594
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C-3 plants. Oecologia 78:9–19
Farquhar GD, von Cammerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Fetcher N, Strain BR, Oberbauer SF (1983) Effects of light regime on growth, leaf morphology and water relations of seedlings of two species of tropical trees. Oecologia 58:314–319
Field CB (1983) Allocating leaf nitrogen for the maximisation of carbon gain: leaf age as a control on the allocation program. Oecologia 56:341–347
Givnish TJ (1986) On the economy of plant form and function. Cambridge University Press, Cambridge
Grimshaw HM, Allen SE, Parkinson JA (1989) Nutrient elements. In: Allen SE, Grimshaw HM, Parkinson JA et al (eds) Chemical analysis of ecological materials. Blackwell, Oxford
Grubb PJ (1998) A reassessment of the strategies of plants which cope with shortages of resources. Perspect Plant Ecol Evol Syst 1:3–31
Hirose T, Werger MJA, Pons TL (1988) Canopy structure and leaf nitrogen distribution in a stand of Lysimachia vulgaris L. as influenced by stand density. Oecologia 77:145–150
Kenzo T, Ichie T, Watanabe Y et al (2006) Changes in photosynthesis and leaf characteristics with tree height in five dipterocarp species in tropical rain forest. Tree Physiol 26:865–873
Kull O, Jarvis PG (1995) The role of nitrogen in a simple scheme to scale-up photosynthesis from leaf to canopy. Plant Cell Envir 18:1174–1182
Lawlor DW (1993) Photosynthesis: molecular, physiological and environmental processes. Longman, London
Lawson GJ (1995) Growth of indigenous tree plantations in the Mbalmayo Forest Reserve, Cameroon. ITE, Edinburgh
Leverenz JW, Jarvis PG (1980) Photosynthesis in Sitka spruce (Picea sitchensis (BONG.) CARR.). X. Acclimation to quantum flux density within and between trees. J Appl Ecol 17:697–708
Levy PE, Moncrieff JB, Massheder JM et al (1997) CO2 fluxes at leaf and canopy scale in millet, fallow and tiger bush vegetation at the HAPEX-Sahel southern super-site. J Hydrol 189:612–632
Lloyd J, Farquhar GD (1994) C-13 discrimination during CO2 assimilation by the terrestrial biosphere. Oecologia 99:201–215
Lloyd J, Bird MI, Veenendaal E et al (2001) Should phosphorus availability be constraining moist tropical forest responses to increasing CO2 concentrations? In: Schulze ED, Harrison SP, Heimann M et al (eds) Global biogeochemical cycles in the climate system. Academic Press, New York
Longman KA, Jeník JA (1987) Tropical forests and their environment, 2nd edn. Longman, Harlow
Martinelli LA, Piccolo MC, Townsend AR et al (1999) Nitrogen stable isotopic composition of leaves and soil: tropical versus temperate forests. Biogeochemistry 46:45–65
Medlyn BE, Badeck FW, De Pury DGG et al (1999) Effects of elevated [CO2] on photosynthesis in European forest species: a meta-analysis of model parameters. Plant Cell Envir 22:1475–1495
Medlyn BE, Dreyer E, Ellsworth D et al (2002) Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Envir 25:1167–1179
Meir P, Grace J, Miranda AC (2001) Leaf respiration in two tropical rain forests: constraints on physiology by phosphorus, nitrogen and temperature. Func Ecol 15:378–387
Meir P, Kruijt B, Broadmeadow M et al (2002) Acclimation of photosynthetic capacity to irradiance in tree canopies in relation to leaf nitrogen concentration and leaf mass per unit area. Plant Cell Envir 25:343–357
Ngeh P (1989) Effects of land clearing methods on a tropical forest ecosystem and the growth of Terminalia ivorensis (A. Chev.). PhD thesis, University of Edinburgh
Niinemets U (1999) Components of leaf dry mass per area—thickness and density—alter leaf photosynthetic capacity in reverse directions in woody plants; research review. New Phyt 144:35–47
Niinemets U, Tenhunen JD (1997) A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Envir 20:845–866
Peterson AG, Ball JT, Luo Yq et al (1999) The photosynthesis leaf nitrogen relationship at ambient and elevated atmospheric carbon dioxide: a meta-analysis. Glob Chan Biol 5:331–346
Poorter L, Bongers F (2006) Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87:1733–1743
Poorter L, de Plassche MV, Willems S et al (2004) Leaf traits and herbivory rates of tropical tree species differing in successional status. Plant Biol 6:746–754
Raaimakers D, Boot RGA, Dijkstra P et al (1995) Photosynthetic rates in relation to leaf phosphorus content in pioneer versus climax tropical rain forest trees. Oecologia 102:120–125
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Nat Acad Sci USA 94:13730–13734
Riddoch I, Grace J, Fasehun FE et al (1991) Photosynthesis and successional status of seedlings in a tropical semideciduous rain-forest in Nigeria. J Ecol 79:491–503
Schulze E-D, Kelliher FM, Körner C et al (1994) Relationships among maximum stomatal conductance, ecosystem surface conductance, carbon assimilation rate and plant nitrogen nutrition: a global ecology scaling exercise. Ann Rev Ecol Syst 25:629–660
Sellers PJ, Berry JA, Collatz GJ et al (1992) Canopy reflectance, photosynthesis, and transpiration. 3. A reanalysis using improved leaf models and a new canopy integration scheme. Rem Sens Envir 42:187–216
Stitt M, Schulze D (1994) Does Rubisco control the rate of photosynthesis and plant growth—an exercise in molecular ecophysiology. Plant Cell Envir 17:465–487
Swaine MD, Whitmore TC (1988) On the definition of ecological species groups in tropical forests. Vegetatio 75:81–86
Taiz L, Zeiger E (2006) Plant physiology, 4th edn. Sinauer, Massachusetts
Takashima T, Hikosaka K, Hirose T (2004) Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Envir 27:1047–1054
Tanner EVJ, Vitousek PM, Cuevas E (1998) Experimental investigation of nutrient limitation of forest growth on wet tropical mountains. Ecology 79:10–22
Vitousek PM (1984) Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 65:285–298
White LP (1970) Brousse tigree patterns in southern Niger. J Ecol 58:549–553
White LP (1971) Vegetation stripes on sheet wash surfaces. J Ecol 59:615–622
Wilson KB, Baldocchi DD, Hanson PJ (2000) Spatial variability of photosynthetic parameters and their relationship to leaf nitrogen in a deciduous forest. Tree Physiol 20:565–578
Worral GA (1960) Tree patterns in the Sudan. J Soil Sci 11:63–67
Wright IJ, Reich PB, Westoby M (2001) Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Func Ecol 15:423–434
Wright IJ, Reich PB, Westoby M et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Wullschleger SD (1993) Biochemical limitations to carbon assimilation in C-3 plants—a retrospective analysis of the A/Ci curves from 109 species. J Exp Bot 44:907–920
Acknowledgements
We would like to thank J. Brouwer and the staff at ICRISAT Sahelian Centre for help during field work. We are grateful to the UK NERC programme ‘Terrestrial Initiative for Global Environment Research’, TIGER (grant no. GST/02/065) and to DFID and ONADEF for financial and infrastructural support in Cameroon. We would also like to thank L. Gormley, J. Lloyd and A. Gray for providing technical and scientific help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meir, P., Levy, P.E., Grace, J. et al. Photosynthetic parameters from two contrasting woody vegetation types in West Africa. Plant Ecol 192, 277–287 (2007). https://doi.org/10.1007/s11258-007-9320-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-007-9320-y