Abstract
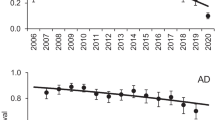
The populations of the ecologically dominant ungulates in the Serengeti ecosystem (zebra, wildebeest and buffalo) have shown markedly different trends since the 1960s: the two ruminants both irrupted after the elimination of rinderpest in 1960, while the zebras have remained stable. The ruminants are resource limited (though parts of the buffalo population have been limited by poaching since the 1980s). The zebras’ resource acquisition tactics should allow them to outcompete the ruminants, but their greater spatial dispersion makes them more available to predators, and it has been suggested that this population is limited by predation. To investigate the mechanisms involved in the population dynamics of Serengeti zebra, we compared population dynamics among the three species using demographic models based on age-class-specific survival and fecundity. The only major difference between zebra and the two ruminants occurred in the first-year survival. We show that wildebeest have a higher reproductive potential than zebra (younger age at first breeding and shorter generation time). Nevertheless, these differences in reproduction cannot account for the observed differences in the population trends between the zebra and the ruminants. On the other hand, among-species differences in first-year survival are great enough to account for the constancy of zebra population size. We conclude that the very low first-year survival of zebra limits this population. We provide new data on predation in the Serengeti and show that, as in other ecosystems, predation rates on zebras are high, so predation could hold the population in a “predator pit”. However, lion and hyena feed principally on adult zebras, and further work is required to discover the process involved in the high mortality of foals.



Similar content being viewed by others
References
Bauer H (2003) Lion conservation in West and Central Africa: integrating social and natural science for wildlife conflict resolution around Waza National Park, Cameroon. PhD thesis, Leiden University, Leiden
Bell RHV (1970) The use of the herd layer by grazing ungulates in the Serengeti. Animal populations in relation to their food resources. Blackwell, Oxford
Berger J (1986) Wild horses of the Great Basin. Social competition and population size. University of Chicago Press, Chicago, Ill.
Caswell H (2001) Matrix population models. Construction, analysis and interpretation. Sinauer, Sunderland, Mass.
Cooper SM (1990) The hunting behaviour of spotted hyaenas (Crocuta crocuta) in a region containing both sedentary and migratory populations of herbivores. Afr J Ecol 28:131–141
Cumming DHM (1982) The influence of large herbivores on savanna structure in Africa. In: Huntley BJ, Walker BH (eds) Ecology of tropical savannas. Springer, Berlin Heidelberg New York, pp 217–244
De Boer W, Prins H (1990) Large herbivores that strive mightily but eat and drink as friends. Oecologia 82:264–274
Dublin HT, Sinclair ARE, Boutin S, Anderson E, Jago M, Arcese P (1990) Does competition regulate ungulate populations? Further evidence from Serengeti, Tanzania. Oecologia 82:283–288
Duncan P, Foose TJ, Gordon I, Gakahu CG, Lloyd M (1990) Comparative nutrient extraction from forages by grazing bovids and equids: a test of the nutritional model of equid/bovid competition and coexistence. Oecologia 84:411–418
Festa-Bianchet M, Gaillard JM, Coté SD (2003) Variable age structure and apparent density-dependence in survival of adult ungulates. J Anim Ecol 72:640–649
Fryxell J, Greever J, Sinclair A (1988) Why are migratory ungulates so abundant? Am Nat 131:781–798
Gaillard J-M, Festa-Bianchet M, Yoccoz NG (1998) Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends Ecol Evol 13:58–63
Gaillard J-M, Festa-Bianchet M, Yoccoz NG, Loison A, Toïgo C (2000) Temporal variation in fitness components and population dynamics of large herbivores. Annu Rev Ecol Syst 31:367–393
Gasaway WC, Gasaway KT, Berry HH (1996) Persistent low densities of plains ungulates in Etosha National Park, Namibia: testing the food-regulating hypothesis. Can J Zool 74:1556–1572
Georgiadis N, Hack M, Turpin K (2003) The influence of rainfall on zebra population dynamics: implications for management. J Appl Ecol 40:125–136
Grimsdell JJR (1973) Age determination of the African buffalo, Syncerus caffer Sparrman. East Afr Wildl J 11:31–54
Gwynne MD, Bell RHV (1968) Selection of vegetation components by grazing ungulates in the Serengeti National Park. Nature 220:390–393
Hairston NG, Smith FE, Slobotkin LB (1960) Community structure, population control, and competition. Am Nat 44:421–425
Hansen RM, Mugambi MM, Bauni SM (1985) Diets and trophic ranking of ungulates of the northern Serengeti. J Wildl Manage 49:823–829
Hofer H, East M (1995) Population dynamics, population size, and the commuting system of Serengeti spotted hyenas. In: Sinclair ARE, Arcese P (eds) Serengeti II: dynamics, management, and conservation of an ecosystem. University of Chicago Press, Chicago, Ill., pp 332–363
Hofer H, Campbell KLI, East ML, Huish SA (1996) The impact of game meat hunting on target and non-target species in the Serengeti. In: Taylor V, Dunstone N (eds) The exploitation of mammal populations. Chapman and Hall, London, pp 117–146
Jolly GM (1969) The treatment of errors in aerial counts of wildlife populations. East Afr Agric For J 34:50–55
Klingel H (1969a) The social organisation and population ecology of the plains zebra (Equus quagga). Zool Afr 4:249–263
Klingel H (1969b) Reproduction in the plains zebra, Equus burchelli boehmi: behaviour and ecological factors. J Reprod Fertil Suppl 6:339–345
Kruuk H (1972) The spotted hyena: a study of predation and social behaviour. University of Chicago Press, Chicago, Ill.
Lebreton JD, Millier C (1982) Modèles dynamiques déterministes en biologie. Masson, Paris, p 208
Legendre S, Clobert J (1995) ULM, a software for conservation and evolutionary biologists. J Appl Stat 22:817–834
Leslie PH (1966) The intrinsic rate of increase and the overlap of successive generations in a population of guillemots (Uria Aalge Pont.). J Anim Ecol 35:291–301
Loison A, Festa-Bianchet M, Gaillard JM, Jorgenson JT, Jullien JM (1999) Age-specific survival in five populations of ungulates: evidence of senescence. Ecology 80:2539–2554
Maddock L (1979) The “migration” and grazing succession. In: Sinclair ARE, Norton-Griffiths M (eds) Serengeti: dynamics of an ecosystem. University of Chicago Press, Chicago, Ill., pp 104–129
Mduma SAR, Sinclair ARE, Hilborn R (1999) Food regulates the Serengeti wildebeest: a 40-year record. J Anim Ecol 68:1101–1122
Ménard C, Duncan P, Fleurance G, Georges J-Y, Lila M (2002) Comparative foraging and nutrition of horses and cattle in European wetlands. J Appl Ecol 39:120–133
Mills MGL, Shenk TM (1992) Predator-prey relationships: the impact of lion predation on wildebeest and zebra populations. J Anim Ecol 61:693–702
Mills MGL, Biggs HC, Whyte IJ (1995) The relationship between rainfall, lion predation and population trends in African herbivores. Wildl Res 22:75–88
Moehlman PD (ed) (2002) Equids: zebras, asses and horses. Status survey and conservation action plan. IUCN/SSC Equid Specialist Group. IUCN, Gland, p 190
Ogutu JO, Owen-Smith N (2003) ENSO, rainfall and temperature influences on extreme population declines among African savanna ungulates. Ecol Lett 6:412–419
Pluhacek J, Bartos L (2000) Male infanticide in captive plains zebra, Equus burchelli. Anim Behav 59:689–694
Prins HHT (1996) Behaviour and ecology of the African buffalo: social inequality and decision making. Chapman and Hall, London
Redfern JV, Grant R, Biggs H, Getz WM (2003) Surface-water constraints on herbivore foraging in the Kruger National Park, South Africa. Ecology 84:2092–2107
Schaller GB (1972) The Serengeti lion. University of Chicago Press, Chicago, Ill.
Sinclair ARE (1974) The natural regulation of buffalo populations in East Africa. IV. The food supply as a regulating factor, and competition. East Afr Wildl J 12:291–311
Sinclair ARE (1977) The African buffalo. A study of resource limitation of populations. University of Chicago Press, Chicago, Ill.
Sinclair ARE (1985) Does interspecific competition or predation shape the African ungulate communities? J Anim Ecol 54:899–918
Sinclair ARE (1997) Fertility control of mammal pests and the conservation of endangered marsupials. Reprod Fertil Dev 9:1–16
Sinclair ARE, Arcese P (1995) Serengeti II: dynamics, management, and conservation of an ecosystem. University of Chicago Press, Chicago, Ill.
Sinclair ARE, Norton-Griffiths M (1979) Serengeti: dynamics of an ecosystem. University of Chicago Press, Chicago
Sinclair ARE, Norton-Griffiths M (1982) Does competition or facilitation regulate migrant ungulate populations in the Serengeti? A test of hypotheses. Oecologia 53:364–369
Sinclair ARE, Dublin HT, Borner M (1985) Population regulation of Serengeti wildebeest: a test of the food hypothesis. Oecologia 65:266–268
Sinclair ARE, Mduma SAR, Arcese P (2000) What determines phenology and synchrony of ungulate breeding in Serengeti? Ecology 81:2100–2111
Smuts GL (1976) Reproduction in the zebra mare Equus burchelli antiquorum from the Kruger National Park. Kodoe 19:89–132
Smuts GL (1978) Interrelations between predators, prey, and their environment. BioScience 28:316–320
Spinage CA (1972) African ungulate life tables. Ecology 53:645–652
Treisman M (1975) Predation and the evolution of gregariousness. I. Models for concealment and evasion. Anim Behav 23:779–800
Van Wieren SE (1996) Digestive strategies in ruminants and non-ruminants. PhD thesis. Agricultural University, Wageningen
Watson RM (1967) The population ecology of the Serengeti wildebeest. PhD thesis. Cambridge University, Cambridge
Watson RM (1969) Reproduction of wildebeest, Connochaetes taurinus albojubatus Thomas, in the Serengeti region, and its significance to conservation. J Reprod Fertil [Suppl] 6:287–310
Acknowledgements
We thank the directors and boards of the Serengeti Wildlife Research Institute and Tanzania National Parks, the park wardens of Serengeti National Park. Without them none of the long-term studies on Serengeti ungulates would have been possible. We thank the many colleagues that have improved the manuscript through their comments on early drafts, particularly N. Owen-Smith, H. Fritz, P. Inchausti, H. Kruuk, H. Klingel and two anonymous referees. This study was supported by the CNRS as part of the France-South Africa Programme International de Coopération Scientifique, Plant-Herbivore Dynamics in Changing Environments.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Calculations for zebra juvenile survival
Survival rates were estimated from the proportions of successive age-classes in the formulas below. As field observations were not done in the same month each year, we compared only samples obtained in 2 successive years (we included the comparison between January and November in 1994). For example, the yearling survival in 1988 was estimated from the juvenile proportion in the 1987 wet season and the yearling proportion in the 1988 wet season. For foal survival, we calculated the expected number of newborns in one year from the number of reproductive females in the previous year and their fecundity. To estimate first-year survival (Table 10), we compared the expected recruitment to the yearling class with the proportion of yearlings observed in the same year (Table 11) .
Rights and permissions
About this article
Cite this article
Grange, S., Duncan, P., Gaillard, JM. et al. What limits the Serengeti zebra population?. Oecologia 140, 523–532 (2004). https://doi.org/10.1007/s00442-004-1567-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1567-6