Abstract
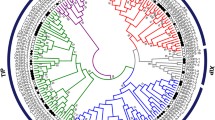
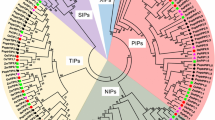
Plant aquaporins are a large and diverse family of water channel proteins that are essential for several physiological processes in living organisms. Numerous studies have linked plant aquaporins with a plethora of processes, such as nutrient acquisition, CO2 transport, plant growth and development, and response to abiotic stresses. However, little is known about this protein family in common bean. Here, we present a genome-wide identification of the aquaporin gene family in common bean (Phaseolus vulgaris L.), a legume crop essential for human nutrition. We identified 41 full-length coding aquaporin sequences in the common bean genome, divided by phylogenetic analysis into five sub-families (PIPs, TIPs, NIPs, SIPs and XIPs). Residues determining substrate specificity of aquaporins (i.e., NPA motifs and ar/R selectivity filter) seem conserved between common bean and other plant species, allowing inference of substrate specificity for these proteins. Thanks to the availability of RNA-sequencing datasets, expression levels in different organs and in leaves of wild and domesticated bean accessions were evaluated. Three aquaporins (PvTIP1;1, PvPIP2;4 and PvPIP1;2) have the overall highest mean expressions, with PvTIP1;1 having the highest expression among all aquaporins. We performed an EST database mining to identify drought-responsive aquaporins in common bean. This analysis showed a significant increase in expression for PvTIP1;1 in drought stress conditions compared to well-watered environments. The pivotal role suggested for PvTIP1;1 in regulating water homeostasis and drought stress response in the common bean should be verified by further field experimentation under drought stress.





Similar content being viewed by others
References
Alexandersson E, Fraysse L, Sjövall-Larsen S, Gustavsson S, Fellert M, Karlsson M, Johanson U, Kjellbom P (2005) Whole gene family expression and drought stress regulation of aquaporins. Plant Mol Biol 59:469–484
Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408:796–815
Aroca R, Ferrante A, Vernieri P, Chrispeels MJ (2006) Drought, abscisic acid and transpiration rate effects on the regulation of PIP gene expression and abundance in Phaseolus vulgaris plants. Ann Bot 98:1301–1310
Beebe SE, Rao IM, Blair MW, Acosta-Gallegos JA (2013) Phenotyping common beans for adaptation to drought. Front Physiol 4:35
Beebo A, Thomas D, Der C, Sanchez L, Leborgne-Castel N, Marty F, Schoefs B, Bouhidel K (2009) Life with and without AtTIP1;1, an Arabidopsis aquaporin preferentially localized in the apposing tonoplasts of adjacent vacuoles. Plant Mol Biol 70:193–209
Beitz E, Wu B, Holm LM, Schultz JE, Zeuthen T (2006) Point mutations in the aromatic arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia and protons. Proc Natl Acad Sci USA 103:269–274
Bello MH, Moghaddam SM, Massoudi M, McClean PE, Cregan PB, Miklas PN (2014) Application of in silico bulked segregant analysis for rapid development of markers linked to Bean common mosaic virus resistance in common bean. BMC Gen 15:903
Bellucci E, Bitocchi E, Ferrarini A, Benazzo A, Biagetti E, Klie S, Minio A, Rau D, Rodriguez M, Panziera A, Venturini L, Attene G, Albertini E, Jackson SA, Nanni L, Fernie AR, Nikoloski Z, Bertorelle G, Delledonne M, Papa R (2014) Decreased nucleotide and expression diversity and modified coexpression patterns characterize domestication in the common bean. Plant Cell 26:1901–1912
Bienert GP, Møller AL, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, Jahn TP (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282:1183–1192
Bienert GP, Bienert MD, Jahn TP, Boutry M, Chaumont F (2011) Solanaceae XIPs are plasma membrane aquaporins that facilitate the transport of many uncharged substrates. Plant J 66:306–317
Bitocchi E, Bellucci E, Giardini A, Rau D, Rodriguez M, Biagetti E, Santilocchi R, Spagnoletti Zeuli P, Gioia T, Logozzo G, Attene G, Nanni L, Papa R (2013) Molecular analysis of the parallel domestication of common bean (Phaseolus vulgaris) in Mesoamerica and the Andes. New Phytol 197:300–313
Bräutigam A, Gowik U (2010) What can next generation sequencing do for you? Next generation sequencing as a valuable tool in plant research. Plant Biol 12:831–841
Broughton WJ, Hernández G, Blair M, Beebe S, Gepts P, Vanderleyden J (2003) Beans (Phaseolus spp.)—model food legumes. Plant Soil 252:55–128
Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R (2001) Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol 152:1206–1215
Chaumont F, Moshelion M, Daniels MJ (2005) Regulation of plant aquaporin activity. Biol Cell 97:746–764
Choi WG, Roberts DM (2007) Arabidopsis NIP2;1:a major intrinsic protein transporter of lactic acid induced by anoxic stress. J Biol Chem 282:24209–24218
Chou KC, Shen HB (2010) Plant-mPLoc: a top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 5:e11335
Cohen D, Bogeat-Triboulot MB, Vialet-Chabrand S, Merret R, Courty PE, Moretti S, Bizet F, Guillot A, Hummel I (2013) Developmental and environmental regulation of aquaporin gene expression across Populus species: divergence or redundancy? PLoS ONE 8:e55506
Cortés AJ, Chavarro MC, Madriñán S, This D, Blair MW (2012a) Molecular ecology and selection in the drought-related Asr gene polymorphisms in wild and cultivated common bean (Phaseolus vulgaris L.). BMC Genet 13:58
Cortés AJ, This D, Chavarro C, Madriñán S, Blair MW (2012b) Nucleotide diversity patterns at the drought-related DREB2 encoding genes in wild and cultivated common bean (Phaseolus vulgaris L.). Theor Appl Genet 125:1069–1085
Cortés AJ, Monserrate FA, Ramirez-Villegas J, Madriñán S, Blair MV (2013) Drought tolerance in wild plant population: the case of common beans (Phaseolus vulgaris L.). PLoS ONE 8:e62898
Dean RM, Rivers RL, Zeidel ML, Roberts DM (1999) Purification and functional reconstitution of soybean nodulin 26. An aquaporin with water and glycerol transport properties. Biochemistry 38:347–353
Fetter K, Van Wilder V, Moshelion M, Chaumonf F (2004) Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 16:215–228
Flagel LE, Wendel JF (2009) Gene duplication and evolutionary novelty in plants. New Phytol 183:557–564
Flexas J, Ribas-Carbó M, Hanson DT, Bota J, Otto B, Cife J, McDowell N, Medrano H, Kaldenhoff R (2006) Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J 48:427–439
Gepts P, Osborn T, Rashka K, Bliss F (1986) Phaseolin-protein variability in wild form and landraces of the common bean (Phaseolus vulgaris): evidence for multiple centers of domestication. Econ Bot 40:451–468
Giorgi FM, Del Fabbro C, Licausi F (2013) Comparative study of RNA-seq- and microarray-derived coexpression networks in Arabidopsis thaliana. Bioinformatics 15:717–724
Gomes D, Agasse A, Thiébaud P, Delrot S, Gerós H, Chaumont F (2009) Aquaporins are multifunctional water and solute transporters highly divergent in living organism. Biochem Biophys Acta 1788:1213–1228
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acid Res 40:D1178–D1186
Guoy M, Guindon S, Gascuel O (2010) SeaView Version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224
Gupta AB, Sankararamakrishnan R (2009) Genome wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa: characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant Biol 9:134
Heckwolf M, Pater D, Hanson DT, Kaldenhoff R (2011) The Arabidopsis thaliana aquaporin AtPIP1:2 is a physiologically relevant CO2 transport facilitator. Plant J 67:734–737
Holm LM, Jahn TP, Møller AL, Schjoerring JK, Ferri D, Klaerke DA, Zeuthen T (2005) NH3 and NH4 + permeability in aquaporin-expressin Xenopus oocytes. Pflugers Arch 450:415–428
Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K (2007) WoLF PSORT: protein localization predictor. Nucleic Acid Res 35:W585–W587
Hub JS, de Groot BL (2008) Mechanisms of selectivity in aquaporins and aquagliceroporins. Proc Natl Acad Sci USA 105:1198–1203
Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjövall S, Fraysse L, Weig AR, Kjellbom P (2001) The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic protein in plants. Plant Physiol 126:1358–1369
Koenig R, Gepts P (1989) Allozyme diversity in wild Phaseolus vulgaris: further evidence for two major centers of diversity. Theor Appl Genet 78:809–817
Koinange EMK, Gepts P (1992) Hybrid weakness in wild Phaseolus vulgaris L. J Hered 83:135–139
Krogh A, Larsson B, von Heijne G, Sonnhammer LL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580
Kwak M, Gepts P (2009) Structure of genetic diversity in the two major gene pools of common bean (Phaseolus vulgaris L., Fabaceae). Theor Appl Genet 118:979–992
Kwak M, Toro O, Debouck D, Gepts P (2012) Multiple origins of the determinate growth habit in domesticated common bean (Phaseolus vulgaris L.). Ann Bot 110:1573–1580
Li G, Santoni V, Maurel C (2014) Plant aquaporins: role in plant physiology. Biochim Biophys Acta 1840:1574–1582
Liu L, Ludewig U, Gasset B, Frommer WB, von Wiren N (2003) Urea transport by nitrogen-regulated tonoplastic intrinsic proteins in Arabidopsis. Plant Physiol 133:1220–1228
Lopez D, Bronner G, Brunel N, Auguin D, Bourgerie S, Brignolas F, Carpin S, Tournaire-Roux C, Maurel C, Fumanal B, Martin F, Sakr S, Label P, Julien JL, Gousset-Dupont A, Venisse JS (2012) Insight into Populus XIP aquaporins: evolutionary expansion, protein functionality and environmental regulation. J Exp Bot 63:2217–2230
Loque D, Ludewig U, Yuan L, von Wiren N (2005) Tonoplastic intrinsic proteins AtTIP2;1 and AtTIP2,3 facilitate NH3 transport into the vacuole. Plant Physiol 137:671–680
Ludevid D, Höfte H, Himelblau E, Chrispeels MJ (1992) The expression pattern of the tonoplastic intrinsic protein γ-TIP in Arabidopsis thaliana is correlated with cell enlargement. Plant Physiol 100:1633–1639
Lynch M, Conery JS (2000) The evolutionary fate and consequences of duplicate genes. Science 290:1151–1155
Ma S, Quist TM, Ulanov A, Joly R, Bohnert HJ (2004) Loss of TIP1;1 aquaporin in Arabidopsis leads to cell and plant death. Plant J 40:845–859
Ma JG, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440:688–691
Magadum S, Banerjee U, Murugan P, Gangapur D, Ravikesavan R (2013) Gene duplication as a major force in evolution. J Genet 92:155–161
Mamidi S, Rossi M, Moghaddam SM, Annam D, Lee R, Papa R, McClean PE (2013) Demographic factors shaped diversity in the two gene pools of wild common bean Phaseolus vulgaris L. Heredity 110:267–276
Maurel C (2007) Plant aquaporins: novel function and regulations properties. FEBS Lett 581:2227–2236
Maurel C, Verdoucq L, Luu DT, Santoni V (2008) Plant aquaporins: membrane channels with multipe integrated functions. Annu Rev Plant Biol 59:595–624
Mitani-Ueno N, Yamaji N, Zhao F, Ma JF (2011) The aromatic/arginine selectivity filter of NIP aquaporins play a critical role in substrate selectivity for silicon, boron and arsenic. J Exp Bot 62:4391–4398
Montalvo-Hernández L, Piedra-Ibarra E, Gómez-Silva L, Lira-Carmona R, Acosta-Gallegos JA, Vazquez-Medrano J, Xoconostle-Cázares B, Ruíz-Medrano R (2008) Differential accumulation of mRNAs in drought-tolerant and susceptible common bean cultivars in response to water deficit. New Phytol 177:102–113
Mori IC, Rhee J, Shibasaka M, Sasano S, Kaneko T, Horie T, Katsuhara M (2014) CO2 transport by PIP2 aquaporins in barley. Plant Cell Physiol 55:251–257
Mukeshimana G, Butare L, Cregan PB, Blair MW, Kelly JD (2014) Quantitative trait loci associated with drought tolerance in common bean. Crop Sci 54:923–938
Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y (2000) Structural determinants of water permeation through aquaporin-1. Nature 407:599–605
Nguyen MX, Moon S, Jung KH (2013) Genome-wide expression analysis of rice aquaporin genes and development of a functional gene network mediated by aquaporin expression in roots. Planta 238:669–681
Peng Y, Lin W, Cai W, Arora R (2007) Overexpression of a Panax ginseng tonoplast aquaporin alters salt tolerance, drougth tolerance and cold acclimation ability in transgenic Arabidopsis plants. Planta 226:729–740
Phillips AL, Huttly AK (1994) Cloning of two gibberellin-regulated cDNAs from Arabidopsis thaliana by subtractive hybridization: expression of the tonoplast water channel, γ-TIP, is increased by GA3. Plant Mol Biol 24:603–615
Quinlan AR, Hall IM (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26:841–842
Recchia GH, Caldas DG, Beraldo AL, da Silva MJ, Tsai SM (2013) Transcriptional analysis of drought-induced genes in the roots of a tolerant genotype in the common bean (Phaseolus vulgaris L.). Int J Mol Sci 14:7155–7179
Repinski SL, Kwak M, Gepts P (2012) The common bean growth habit gene PvTFL1y is a functional homolog of Arabidopsis TFL1. Theor Appl Genet 124:1539–1547
Reuscher S, Akiyama M, Mori C, Aoki K, Shibata D (2013) Genome-wide identification and expression analysis of aquaporins in tomato. PLoS ONE 8:e79052
Rice P, Longden I, Bleasby A (2000) EMBOSS: the european molecular biology open software suite. Trends Genet 16:276–277
Schmutz J, McClean PE, Mamidi S, Wu GA, Cannon SB, Grimwood J, Jenkins J, Shu S, Song Q, Chavarro C, Torres-Torres M, Geffroy V, Moghaddam SM, Gao D, Abernathy B, Barry K, Blair M, Brick MA, Chovatia M, Gepts P, Goodstein DM, Gonzales M, Hellsten U, Hyten DL, Jia G, Kelly JD, Kudrna D, Lee R, Richard MM, Miklas PN, Osorno JM, Rodrigues J, Thareau V, Urrea CA, Wang M, Yu Y, Zhang M, Wing RA, Cregan PB, Rokhsar DS, Jackson SA (2014) A reference genome for common bean and genome-wide analysis of dual domestication. Nat Genet 46:707–713
Schüssler MD, Alexandersson E, Bienert GP, Kichey T, Laursen KH, Johanson U, Kjellbom P, Schjoerring JK, Jahn TP (2008) The effects of the loss of TIP1;1 and TIP1;2 aquaporins in Arabidopsis thaliana. Plant J 56:756–767
Severing EI, Van Dijk ADJ, Stiekema WJ, Van Ham RCHJ (2009) Comparative analysis indicates that alternative splicing in plants has a limited role in functional expansion of the proteome. BMC Genom 10:154
Takano J, Wada M, Ludewig U, Schaaf G, von Wirén N, Fujiwara T (2006) The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18:1498–1509
Tao P, Zhong X, Li B, Wang W, Yue Z, Lei J, Guo W, Huang X (2014) Genome-wide identification and characterization of aquaporin genes (AQP) in chinese cabbage (Brassica rapa spp. pekinensis). Mol Genet Genomics doi:10.1007/s00438-014-0874-9
Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovery splice junctions with RNA-Seq. Bioinformatics 25:1105–1111
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515
Usadel B, Obayashi T, Mutwil M, Giorgi FM, Bassel GW, Tanimoto M, Chow A, Steinhauser D, Persson S, Provart NJ (2009) Co-expression tools for plant biology: opportunities for hypothesis generation and caveats. Plant, Cell Environ 32:1633–1651
Wallace IS, Roberts DM (2005) Distinct transport selectivity of two structural subclasses of the nodulin-like intrinsic protein family of plant aquaglyceroporin channels. Biochemistry 44:16826–16834
Wallace IS, Choi W, Roberts DM (2006) The structure, function and regulation of the Nodulin 26-like intrinsic protein family of plant aquaglyceroporins. Biochim Biophys Acta 1758:1165–1175
Xulvi-Brunet R, Li H (2010) Co-expression networks: graph properties and topological comparisons. Bioinformatics 26:205–214
Zelazny E, Borst JW, Muyalaert M, Batoko H, Hemminga Chaumont F (2007) FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proc Natl Acad Sci USA 104:12359–12364
Zelazny E, Miecielica U, Borst JW, Hemminga MA, Chaumont F (2009) An N-terminal diacidic motif is required for the trafficking of maize aquaporins ZmPIP2;4 and ZMPIP2;5 to the plasma membrane. Plant J 57:346–355
Zhang DY, Ali Z, Wang CB, Xu L, Yi JX, Xu ZL, Liu XQ, He XL, Huang YH, Khan IA, Trethowan RM, Ma HX (2013) Genome-wide sequence characterization and expression analysis of major intrinsic proteins in soybean (Glycine max L.). PLoS ONE 8:e56312
Zhu C, Schraut D, Hartung W, Schaffner AR (2005) Differential responses of maize MIP genes to salt stress and ABA. J Exp Bot 56:2971–2981
Zhu Y, Stephens RM, Meltzer PS, Davis SR (2013) SRAdb: query and use public next-generation sequencing data from within R. BMC Bioinf 14:19
Acknowledgments
This project was supported by Agriculture and Food Research Initiative (AFRI) Competitive Grant No. 2013-67013-21224 from the USDA National Institute of Food and Agriculture.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Hohmann.
Electronic supplementary material
Below is the link to the electronic supplementary material.
438_2015_1038_MOESM1_ESM.pdf
Supplementary material 1 (PDF 11075 kb). Supplementary File S1 Multiple sequence alignment of the first NPA region in the putative aquaporins of P. vulgaris. Phytozome jbrowse RNA-Sequencing log-coverage link and Phytomine FPKM expression link refers to link of RNA-Sequencing coverage and FPKM expression of Phvul.003G040100
438_2015_1038_MOESM2_ESM.pdf
Supplementary material 2 (PDF 21 kb). Supplementary File S2 FPKM expression of PvPIP1;4 in the different organs and genotypes analyzed
438_2015_1038_MOESM3_ESM.pdf
Supplementary material 3 (PDF 12 kb). Supplementary File S3 Pairwise protein alignment of the putative tandemly duplicated aquaporins
438_2015_1038_MOESM4_ESM.pdf
Supplementary material 4 (PDF 716 kb). Supplementary File S4 Expression heatmap of the 41 aquaporins in different domesticated and wild common bean genotypes. The expression values of each aquaporins are log2 transformed
438_2015_1038_MOESM5_ESM.pdf
Supplementary material 5 (PDF 24 kb). Supplementary File S5 Putative interacting aquaporins with a Pearson’s correlation coefficient (r) > 0.9
438_2015_1038_MOESM6_ESM.pdf
Supplementary material 6 (PDF 19 kb). Supplementary File S6 Aquaporin normalized expression and expression-fold change under drought stress and in background ESTs. P value, based on 2 × 2 χ2 tests, is shown
438_2015_1038_MOESM7_ESM.pdf
Supplementary material 7 (PDF 20 kb). Supplementary File S7 Summary of BARCBean6K_3 SNPs that have an aquaporin gene locus as the nearest annotated genomic feature. Distance between the markers and the aquaporins, as well as genomic positions of both, is shown
438_2015_1038_MOESM8_ESM.pdf
Supplementary material 8 (PDF 22 kb). Supplementary File S8 Distance and genomic positions of the drought QTLs identified by Mukeshimana et al. (2014) and the nearest annotated aquaporins
Rights and permissions
About this article
Cite this article
Ariani, A., Gepts, P. Genome-wide identification and characterization of aquaporin gene family in common bean (Phaseolus vulgaris L.). Mol Genet Genomics 290, 1771–1785 (2015). https://doi.org/10.1007/s00438-015-1038-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-015-1038-2