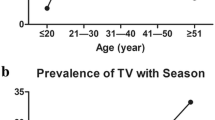Abstract
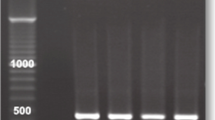
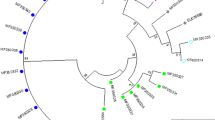
Trichomoniasis is a common human sexually transmitted infection caused by Trichomonas vaginalis. The parasite can be infected with double-stranded RNA viruses (TVV). This viral infection may have important implications on trichomonal virulence and disease pathogenesis. This study aimed to determine the prevalence of T. vaginalis virus among isolates obtained from infected (symptomatic and asymptomatic) women in Ismailia City, Egypt, and to correlate the virus-infected isolates with the clinical manifestations of patients. In addition, the pathogenicity of TVV infected isolates on mice was also evaluated. T. vaginalis isolates were obtained from symptomatic and asymptomatic female patients followed by axenic cultivation in Diamond’s TYM medium. The presence of T. vaginalis virus was determined from total extraction of nucleic acids (DNA-RNA) followed by reverse transcriptase-PCR. Representative samples were inoculated intraperitoneally in female albino/BALB mice to assess the pathogenicity of different isolates. A total of 110 women were examined; 40 (36.3 %) samples were positive for T. vaginalis infection. Of these 40 isolates, 8 (20 %) were infected by TVV. Five isolates contained TVV-2 virus species, and the remaining three isolates were infected withTVV-4 variant. A significant association was found between the presence of TVV and particular clinical manifestations of trichomoniasis. Experimental mice infection showed varying degrees of pathogenicity. This is the first report on T. vaginalis infection by TVV in Egypt. The strong association detected between TVV and particular clinical features of trichomoniasis and also the degree of pathogenicity in experimentally infected mice may indicate a possible clinical significance of TVV infection of T. vaginalis isolates.



Similar content being viewed by others
References
Alderete JF, Kasmala L, Metcalfe E, Garza GE (1986) Phenotypic variation and diversity among Trichomonas vaginalis isolates and correlation of phenotype with trichomonal virulence determinants. Infect Immun 53(2):285–293
Alderete J, Demas P, Gombosova A, Valent M, Yanoska A, Fabusova H, Metcalfe E (1987) Phenotypes and protein-epitope phenotypic variation among fresh isolates of Trichomonas vaginalis. Infect Immun 55(5):1037–1041
Alderete JF, Arroyo R, Dailey DC, Engbring J, Khoshnan MA, Lehker MW, McKay J (1992) Molecular analysis of Trichomonas vaginalis surface protein repertoires. Mol Cell Biol Hum Dis Ser 1:173–202
Arroyo R, Alderete J (1994) Two Trichomonas vaginalis surface proteinases bind to host epithelial cells and are related to levels of cytoadherence and cytotoxicity. Arch Med Res 26(3):279–285
Benchimol M, Monteiro S, Chang TH, Alderete JF (2002) Virus in Trichomonas—an ultrastructural study. Parasitol Int 51(3):293–298
Bessarab IN, Nakajima R, Liu HW, Tai JH (2011) Identification and characterization of a type III Trichomonas vaginalis virus in the protozoan pathogen Trichomonas vaginalis. ArchVirol 156(2):285–294
Bhatt R, Deodhar L, Pandit D, Bhise R, Chatterjee D (1997) Comparative pathogenicity of Trichomonas vaginalis isolated from symptomatic and asymptomatic cases. J Postgrad Med 43(3):68
Boulos LM, El-Temsahy MM, Aly SM, El agamy EI, Amerf EI (2012) Biological and biochemical studies for characterization of some egyptian Trichomonas vagianlis isolates. PUJ 5(2):175-188
Caterina P, Warton A, Papadimitriou J, Ashman R (1996) Relationship of the virulence of Trichomonas vaginalis and the major histocompatibility complex in murine trichomonad infection. Parasitology Res 82(7):628–633
Champney WS, Curti SK, Samuels R (1995) Cytopathology and release of an RNA virus from a strain of Trichomonas vaginalis. Int JParasitol 25(12):1463–1471
Chesson HW, Blandford JM, Pinkerton SD (2004) Estimates of the annual number and cost of new HIV infections among women attributable to trichomoniasis in the United States. Sex Trans Dis 31(9):547–551
Cotch MF, Joseph P, Nugent I, Hillier SL, Gibbs RS, Martin DH, Regan JA (1997) Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex Trans Dis 24(6):353–360
Diamond LS, Mattern CF, Bartgis IL (1972) Viruses of Entamoeba histolytica I. Identification of transmissible virus-like agents. J virolo 9(2):326–341
Diamond LS, Harlow DR, Cunnick CC (1978) A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg 72(4):431–432
El-Moamly A, Rashad S (2008) Trichomonas vaginalis antigens in vaginal and urine specimens by immunochromatography, compared to culture and microscopy. J Egypt Soc Parasitol 38(2):573–584
Fichorova RN, Lee Y, Yamamoto HS, Takagi Y, Hayes GR, Goodman RP, Kula T (2012) Endobiont viruses sensed by the human host—beyond conventional antiparasitic therapy. PLoS One 7, e48418
Fraga J, Rojas L, Sariego I, Fernandez-Calienes A (2005) Double-stranded RNA viral infection in Cuban Trichomonas vaginalis isolates. Braz J Infect Dis 9(6):521–524
Fraga J, Rojas L, Sariego I, Fernandez-Calienes A, Nunez FA (2007) Double-stranded RNA viral infection of Trichomonas vaginalis and association with clinical presentation. Acta protozool 46(2):93
Fraga J, Rojas L, Sariego I, Fernandez-Calienes A (2012) Genetic characterization of three Cuban Trichomonas vaginalis virus. Phylogeny of Totiviridae family. Infect Genet Evol 12(1):113–120
Gerhold RW, Allison AB, Sellers H, Linnemann E, Alderete JF, Chang TH (2009) Examination for double-stranded RNA viruses in Trichomonas gallinae and identification of a novel sequence of a Trichomonas vaginalis virus. Parasitol Res 105(3):775–779
Gómez-Barrio A, Nogal-Ruiz JJ, Montero-Pereira D, Rodríguez-Gallego E, Romero-Fernández E, Escario JA (2002) Biological variability in clinical isolates of Trichomonas vaginalis. Mem Inst Oswaldo Cruz 97(6):893–896
Goodman RP, Freret TS, Kula T, Geller AM, Talkington MW, Tang-Fernandez V, Beach DH (2011a) Clinical isolates of Trichomonas vaginalis concurrently infected by strains of up to four Trichomonasvirus species (family Totiviridae). J Virol 85(9):4258–4270
Goodman RP, Ghabrial SA, Fichorova RN, Nibert ML (2011b) Trichomonasvirus: a new genus of protozoan viruses in the family Totiviridae. Arch Virol 156(1):171–179
Hampl V, Vaňáčová Š, Kulda J, Flegr J (2001) Concordance between genetic relatedness and phenotypic similarities of Trichomonas vaginalis strains. BMC Evol Biol 1(1):11
Hegazi M, Elbahey M, Makhlouf L, El-Hamshary E, Dawoud H, El-Gayar E (2009) Polymerase chain reaction versus conventional methods in the diagnosis of vaginal trichomoniasis. J Egypt Soc Parasitol 39(1):11–21
Heidary S, Bandehpour M, Valadkhani Z, Seyyed-Tabaee S, Haghighi A, Abadi A, Kazemi B (2013) Double-stranded RNA viral infection in Tehran Trichomonas vaginalis isolates. Iran J Parasitol 8(1):60–64
Helms DJ, Mosure DJ, Metcalf CA, Douglas JM Jr, Malotte CK, PaulS M, Peterman TA (2008) Risk factors for prevalent and incident Trichomonas vaginalis among women attending three sexually transmitted disease clinics. Sex Trans Dis 35(5):484–488
Honigberg B (1978) Trichomonads of importance in human medicine. Parasitic protozoa 2(7):275–279
Hussien E, El-Sayed H, Shaban M, Salm A, Rashwan M (2004) Biological variability of Trichomonas vaginalis clinical isolates from symptomatic and asymptomatic patients. J Egypt Soc Parasitol 34(3):979–988
Ives A, Ronet C, Prevel F, Fuertes-Marraco S, Ruzzante G, Schutz F, Hickerson SM (2011) Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science 331(6018):775–778
Kaur S, Khurana S, Bagga R, Wanchu A, Malla N (2008) Antitrichomonas IgG, IgM, IgA, and IgG subclass responses in human intravaginal trichomoniasis. ParasitolRes 103(2):305–312
Kengne P, Veas F, Vidal N, Rey YL, CUNY G (1994) Specific polymerase chain reaction diagnosis. Cell Mol Biol 40(6):819–831
Khoshnan A, Alderete J (1994) Trichomonas vaginalis with a double-stranded RNA virus has upregulated levels of phenotypically variable immunogen mRNA. J Virol 68(6):4035–4038
Khramtsov NV, Woods KM, Nesterenko MV, Dykstra CC, Upton SJ (1997) Virus-like, double-stranded RNAs in the parasitic protozoan Cryptosporidium parvum. Mol Microbiol 26(2):289–300
Kim JW, Chung PR, Hwang MK, Choi EY (2007) Double-stranded RNA virus in Korean isolate IH-2 of Trichomonas vaginalis. The KoreanJ Parasitol 45(2):87–94
Kulda J (1990) Employment of experimental animals in studies of Trichomonas vaginalis infection. In: Honigberg BM (ed) Trichomonads parasitic in humans. Springer, New York, pp 112–154. doi:10.1007/978-1-4612-3224-7_8
Malla N, Valadkhani Z, Harjai K, Gupta I, Sharma S (2004) Reactive nitrogen intermediates in experimental trichomoniasis induced with isolates from symptomatic and asymptomatic women. Parasitol Res 94(2):101–105
McClelland RS, Sangaré L, Hassan WM, Lavreys L, Mandaliya K, Kiarie J, Baeten JM (2007) Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis 195(5):698–702
Mead J, Fernadez M, Romagnoli P, Secor W (2006) Use of Trichomonas vaginalis clinical isolates to evaluate correlation of gene expression and metronidazole resistance. J Parasitol 92(1):196–199
Nogal-Ruiz JJ, Escario JA, Martinez RA, Gomez BA (1997) Evaluation of a murine model of experimental trichomoniasis. Parasite 2:127–132
Nogal-Ruiz J, Gomez-Barrio A, Escario J, Martinez-Fernandez A (2003) Effect of Anapsos® in a murine model of experimental trichomoniasis. Parasite 10(4):303–308
Pararas M, Skevaki C, Kafetzis D (2006) Preterm birth due to maternal infection: causative pathogens and modes of prevention. Eur J Clin Microbiol Infect Dis 25(9):562–569
Petrin D, Delgaty K, Bhatt R, Garber G (1998) Clinical and microbiological aspects of Trichomonas vaginalis. Clin MicrobiolRev 11(2):300–317
Provenzano D, Khoshnan A, Alderete J (1997) Involvement of dsRNA virus in the protein compositionand growth kinetics of host Trichomonas vaginalis. Archof Virol 142(5):939–952
Revets H, Dekegel D, Deleersnijder W, De Jonckheere J, Peeters J, Leysen E, Hamers R (1989) Identification of virus-like particles in Eimeria stiedae. Mol Bioch Parasitol 36(3):209–215
Ruiz JN, Escario J, Diaz RM, Barrio AG (1997) Evaluation of a murine model of experimental trichomoniasis. Parasite 4(2):127–132
Ryu JS, Chung HL, Min DY, Cho YH, Ro YS, Kim SR (1999) Diagnosis of trichomoniasis by polymerase chain reaction. Yonsei Med J 40:56–60
Scott P (2011) Leishmania—a parasitized parasite. N Engl J Med 364(18):1773–1774
Snipes LJ, Gamard PM, Narcisi EM, Beard CB, Lehmann T, Secor WE (2000) Molecular epidemiology of metronidazole resistance in a population of Trichomonas vaginalis clinical isolates. J Clin Microbiol 38(8):3004–3009
Stark JR, Alderete JF, Judson G, Mundodi V, Kucknoor AS, Giovannucci EL, Kurth T (2009) Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians’ Health Study. J Natl Cancer Inst 101(20):1406–1411
Stuart KD, Weeks R, Guilbride L, Myler PJ (1992) Molecular organization of Leishmania RNA virus 1. Proc Natl Acad Sci 89(18):8596–8600
Sutcliffe S, Giovannucci E, Alderete JF, Chang TH, Gaydos CA, Zenilman JM, Platz EA (2006) Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 15(5):939–945
Tarr PI, Aline RF, Smiley BL, Scholler J, Keithly J, Stuart K (1988) LR1: a candidate RNA virus of Leishmania. Proc Natl Acad Sci 85(24):9572–9575
Twu O, Dessí D, Vu A, Mercer F, Stevens GC, De Miguel N, Fiori PL (2014) Trichomonas vaginalis homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness, and inflammatory responses. Proc Natl Acad Sci 111(22):8179–8184
Wang AL, Wang CC (1986) The double-stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc Natl Acad Sci U S A 83(20):7956–7960
Wang A, WangC C, Alderete JF (1987) Trichomonas vaginalis phenotypic variation occurs only among trichomonads infected with the double-stranded RNA virus. JExp Med 166(1):142–150
Wang CC, McClelland RS, Reilly M, Overbaugh J, Emery SR, Mandaliya K, Kreiss JK (2001) The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J Infec Dis 183(7):1017–1022
Weber B, Mapeka TM, Maahlo MA, Hoosen AA (2003) Double stranded RNA virus in South African Trichomonas vaginalis isolates. J Clin Pathol 56(7):542–543
Wendel KA, Rompalo AM, Erbelding EJ, Alderete JF, Chang TH (2002) Double-stranded RNA viral infection of Trichomonas vaginalis infecting patients attending a sexually transmitted diseases clinic. J Infect Dis 186(4):558–56
World Health Organization (2012) Global incidence and prevalence of selected curable sexually transmitted infections—2008: World Health Organization. Available at: http://www.who.int/reproductivehealth/publications/rtis/stisestimates/en/; http://apps.who.int/iris/bitstream/10665/75181/1/9789241503839_eng.pdf?ua=1
Yadav M, Gupta I, Malla N (2005) Kinetics of immunoglobulin G, M, A and IgG subclass responses in experimental intravaginal trichomoniasis: prominence of IgG1 response. Parasite Immunol 27(12):461–467
Yadav M, Dubey M, Gupta I, Bhatti G, Malla N (2007) Cysteine proteinase 30 in clinical isolates of T. vaginalis from symptomatic and asymptomatic infected women. Expparasitol 116(4):399–406
Acknowledgments
The authors thank Dr. Maria D. Esteve-Gassent, assistant professor of Veterinary Pathobiology, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, for revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Authorship
Assistant Professor Eman K. El-Gayar have chosen the research idea and planned the study design, shared in the experimental studies, and wrote the manuscript. Dr. Amira B. Mokhtar shared in the study design, the experimental studies, and manuscript writing. Dr. Wael A. Hassan assessed the histopathological results and shared in writing the manuscript.
Ethical considerations
The experimental animal studies were conducted in accordance with the international valid guidelines, and they were maintained under convenient conditions. The research was approved by the Scientific Research Ethical Committee, Faculty of Medicine, Suez Canal University. Informed consents were taken from patients to use their samples in the study.
Sources of funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Rights and permissions
About this article
Cite this article
El-Gayar, E.K., Mokhtar, A.B. & Hassan, W.A. Molecular characterization of double-stranded RNA virus in Trichomonas vaginalis Egyptian isolates and its association with pathogenicity. Parasitol Res 115, 4027–4036 (2016). https://doi.org/10.1007/s00436-016-5174-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5174-3