Abstract
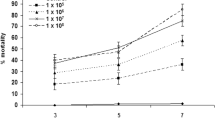
Pr1 is a subtilisin-like protease produced by Metarhizium spp. entomopathogenic fungi, and it is recognized as heavily involved in the initial steps of the fungal invasion of arthropod-host cuticles. In the current study, correlation was sought between mortality of tick larvae and conidial Pr1 levels of one Metarhizium anisopliae senso latu (s.l.) isolate (CG 148). Conidia with different levels of pr1 gene expression and enzymatic activity were obtained by producing them on either artificial medium (to yield low Pr1 activity) or on Rhipicephalus microplus cadavers (to yield high Pr1 activity). Conidial proteolytic activity was assessed using N-suc-ala-ala-pro-phe-ρNA as the chromogenic substrate, and pr1 expression was profiled by qPCR using three genes (gpd, try, and tef) as reference genes. Pr1 enzymatic (proteolytic) activity on conidia obtained from tick cadavers was 36 U mg−1 in comparison to 4 U mg−1 on conidia from PDA medium. Also, pr1 gene expression level was ten times higher in conidia from tick cadavers compared to PDA medium. Bioassays of M. anisopliae s.l. CG 148 spores with elevated Pr1 proteolytic activity and gene expression levels did not demonstrate increased virulence (= significant change percent mortality of tick larvae). The minimal levels of Pr1 on conidia produced on artificial medium was adequate to afford high levels of virulence, and the elevated amounts of the enzyme on tick-cadaver-produced conidia did not induce elevated larval mortality. As long as some Pr1 activity was present, fungal virulence of isolate CG 148 against tick larvae was not elevated by increased levels of conidial Pr1.

Similar content being viewed by others
References
Alves SB (1998) Fungos entomopatogênicos. In: Alves SB (ed) Controle microbiano de insetos. Fundação de Estudos Agrário Luiz de Queirós (FEALQ), Piracicaba, pp 289–382
Angelo IC, Fernandes EKK, Bahiense TC, Perinotto WMS, Moraes APR, Terra ALM (2010) Efficiency of Lecanicillium lecanii to control the tick Rhipicephalus microplus. Vet Parasitol 172:317–322
Bischoff JF, Rehner SA, Humber RA (2009) A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 101:512–530
Bittencourt VREP, Massard CL, Lima AF (1992) Uso do fungo Metarhizium anisopliae (Metschnikoff, 1879) Sorokin, 1883, no controle do carrapato Boophilus microplus (Canestrini, 1887). Arquivo da Universidade Rural do Rio de Janeiro 15:197–202
Bittencourt VREP, Massard CL, Lima AF (1994) Ação do fungo Metarhizium anisopliae sobre a fase não parasitária do ciclo biológico de Boophilus microplus. Revista Universidade Rural, Série Ciências da Vida 16:49–55
Bittencourt VREP, Massard CL, Lima AF (1995) Dinâmica da infecção do fungo Metarhizium anisopliae (Metschnikoff, 1879) Sorokin, 1883, sobre o carrapato Boophilus microplus (Canestrini, 1887). Revista da Universidade Rural, Série Ciências da Vida 17:83–88
Bittencourt VREP, Mascarenhas AG, Faccini JLH (1999) Mecanismo de infecção do fungo Metarhizium anisopliae no carrapato Boophilus microplus em condições experimentais. Ciência Rural, Santa Maria 29:351–354
Camargo MG, Golo PS, Angelo IC, Perinotto WMS, Sá FA, Quinelato S, Bittencourt VREP (2012) Effect of oil-based formulations of acaripathogenic fungi to control Rhipicephalus microplus ticks under laboratory conditions. Vet Parasitol 188:140–147
Campos RA, Arruda W, Boldo JT, Silva MV, Barros NM, Azevedo JL, Schrank A, Vainstain MH (2005) Boophilus microplus infection by Beauveria amorpha and Beauveria bassiana: SEM analysis and regulation of subtilizin-like proteases and chitinases. Cur Microbiol 50:257–261
Castro ABA, Bittencourt VREP, Daemon E, Viegas EC (1997) Eficácia do fungo Metarhizium anisopliae sobre o carrapato Boophilus microplus em teste de estábulo. Revista Universidade Rural, Série Ciências da Vida 19:73–82
Chandler D, Davidson G, Pell JK, Ball BV, Shaw K, Sunderland KD (2000) Fungal biocontrol of Acari. Biocontrol Sci Techn 10:357–384
Costa GL, Sarquis MIM, Moraes AML, Bittencourt VREP (2002) Isolation of Beauveria bassiana and Metarhizium anisopliae var. anisopliae from Boophilus microplus tick (Canestrini, 1887), in Rio de Janeiro State, Brazil. Mycopathol 154:207–209
Dhar P, Kaur G (2010) Cuticle-degrading proteases produced by Metarhizium anisopliae and their induction in different media. Indian J Microbiol 50:449–455
Fang W, Bidochka MJ (2006) Expression of genes involved in germination, conidiogenesis and pathogenesis in Metarhizium anisopliae using quantitative real-time RT-PCR. Mycol Res 110:1165–1171
Fargues JF, Robert PH (1983) Effects of passing through scarabeid hosts on virulence and host specificity of two strains of the entomopathogenic hyphomycete Metarhizium anisopliae. Can J Microbiol 29:576–583
Fernandes EKK, Bittencourt VREP (2008) Entomopathogenic fungi against South American tick species. Exp Appl Acarol 46:71–93
Fernandes EKK, Keyser CA, Rangel DEN, Foster RN, Roberts DW (2010) CTC medium: a novel dodine-free selective medium for isolating entomopathogenic fungi, especially Metarhizium acridum, from soil. Biol Control 54:197–205
Fernandes EKK, Angelo IC, Rangel DEN, Bahiense TC, Moraes AML, Roberts DW, Bittencourt VREP (2011) An intensive search for promising fungal biological control agents of ticks, particularly Rhipicephalus microplus. Vet Parasitol 182:307–318
Fernandes EKK, Bittencourt VREP, Roberts DW (2012) Perspectives on the potential of entomopathogenic fungi in biological control of ticks. Exp Parasitol 130:300–305
Johns R, Sonenshine DE, Hynes WL (1998) Control of bacterial infections in the hard tick Dermacentor variabilis (Acari: Ixodidae): evidence of antimicrobial proteins in tick hemolymph. J Med Entomol 35:458–464
Kawakami K (1960) On the changes of characteristics of the silkworm muscardines through successive cultures. Bull Seric Exp Stn Japan 83-99
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Perinotto WMS, Terra ALM, Angelo IC, Fernandes EKK, Golo PS, Camargo MG, Bittencourt VREP (2012) Nomuraea rileyi as biological control agents of Rhipicephalus microplus tick. Parasitol Res 111:1743–1748
Quinelato S, Golo PS, Perinotto WMS, Sá FA, Camargo MG, Angelo IC, Moraes AML, Bittencourt VREP (2012) Virulence potential of Metarhizium anisopliae s.l. isolates on Rhipicephalus (Boophilus) microplus larvae. Vet Parasitol 190:556–565
Roberts DW, St Leger RJ (2004) Metarhizium spp., cosmopolitan insect pathogenic fungi: mycological aspects. Adv Appl Microbiol 54:1–70
Rosas-García NM, Ávalos-de-León O, Villegas-Mendoza JM, Mireles-Marínez M, Barboza-Corona JE, Castañeda-Ramírez JC (2014) Correlation between pr1 and pr2 gene content and virulence in Metarhizium anisopliae strains. J Microbiol Biotechnol 24:1495–1502
Samish M, Rehacek J (1999) Pathogens and predators of ticks and their potential in biological control. Annu Rev Entomol 44:159–182
Samish M, Ginsberg H, Glaser I (2004) Biological control of ticks. Parasitology 129:S389–S403
Sampaio IBM (2002) Estatística aplicada à experimentação animal. Fundação de Estudo e Pesquisa em Medicina Veterinária e Zootecnia (FEPMVZ-Editora), Belo Horizonte
Santi L, Silva OB, Berger M, Guimarães JA, Schrank A, Vainstein MH (2010) Conidial surface proteins of Metarhizium anisopliae: source of activities related with toxic effects, host penetration and pathogenesis. Toxicon 55:874–880
Sasan RK, Bidochka MJ (2012) The insect-pathogenic fungus Metarhizium robertsii (clavicipitaceae) is also an endophyte that stimulates plant root development. Am J Bot 99:101–107
Schrank A, Vainstein MH (2010) Metarhizium anisopliae enzymes and toxins. Toxicon 56:1267–1274
Small CN, Bidochka MJ (2005) Up-regulation of Pr1, a subtilisin-like protease, during conidiation in the insect pathogen Metarhizium anisopliae. Mycol Res 109:307–313
St Leger RJ, Charnley AK, Cooper RM (1986) Cuticle-degrading enzymes of entomopathogenic fungi: synthesis in culture on cuticle. J Invertebr Pathol 48:85–95
St Leger RJ, Durrand PK, Cooper RM, Charnley AK (1988) Regulation of production of proteolytic enzymes by the entomopathogenic fungus Metarhizium anisopliae. Arch Microbiol 150:413–416
St Leger RJ, Butt TM, Staples RC, Roberts DW (1989) Synthesis of proteins including a cuticle-degrading protease during differentiation of the entomopathogenic fungus Metarhizium anisopliae. Exp Mycol 13:253–262
St Leger RJ, Roberts DW, Staples RC (1991a) A model to explain differentiation of appressoria by germilings of Metarhizium anisopliae. J Invertebr Pathol 57:299–310
St Leger RJ, Goettel M, Roberts DW, Staples RC (1991b) Prepenetration events during infection of host cuticle by Metarhizium anisopliae. J Invertebr Pathol 58:168–179
Tulloch M (1976) The genus Metarhizium. Trans Br Mycol Soc 66:407–411
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol Res 3:0034.1–0034.11
Wang S, Leclerque A, Pava-Ripoll M, Fang W, St Leger RJ (2009) Comparative genomics using microarrays reveals divergence and loss of virulence-associated genes in host-specific strains of the insect pathogen Metarhizium anisopliae. Eukariotic Cell 8:888–898
Zimmermann G (2007) Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci Techn 17:879–920
Acknowledgments
This research was supported by grants from the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq) of Brazil and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) from Rio de Janeiro State, Brazil. We thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and CNPq for providing graduation scholarships for Patrícia S. Golo, Wendell M.S. Perinotto, Mariana G. Camargo, and Fillipe A. Sá. Vânia R.E.P. Bittencourt and Carlos L. Massard are CNPq researchers. We also very much appreciate support from USDA/APHIS (Animal Plant Health and Inspection Service), particularly Dr. Larry E. Jech and Dr. R. Nelson Foster, during the data interpretation and writing phases of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Golo, P.S., Santos, H.A., Perinotto, W.M.S. et al. The influence of conidial Pr1 protease on pathogenicity potential of Metarhizium anisopliae senso latu to ticks. Parasitol Res 114, 2309–2315 (2015). https://doi.org/10.1007/s00436-015-4426-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4426-y