Abstract
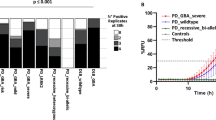
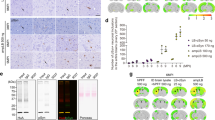
α-Synuclein aggregation underlies pathological changes in Lewy body dementia. Recent studies highlight structural variabilities associated with α-synuclein aggregates in patient populations. Here, we develop a quantitative real-time quaking-induced conversion (qRT-QuIC) assay to measure permissive α-synuclein fibril-templating activity in tissues and cerebrospinal fluid (CSF). The assay is anchored through reference panels of stabilized ultra-short fibril particles. In humanized α-synuclein transgenic mice, qRT-QuIC identifies differential levels of fibril activity across the brain months before the deposition of phosphorylated α-synuclein in susceptible neurons. α-Synuclein fibril activity in cortical brain extracts from dementia with Lewy bodies (DLB) correlates with activity in matched ventricular CSF. Elevated α-synuclein fibril activity in CSF corresponds to reduced survival in DLB. α-Synuclein fibril particles amplified from cases with high fibril activity show superior templating in the formation of new inclusions in neurons relative to the same number of fibril particles amplified from DLB cases with low fibril activity. Our results highlight a previously unknown broad heterogeneity of fibril-templating activities in DLB that may contribute to disease phenotypes. We predict that quantitative assessments of fibril activities in CSF that correlate to fibril activities in brain tissue will help stratify patient populations as well as measure therapeutic responses to facilitate the development of α-synuclein-targeted therapeutics.







Similar content being viewed by others
References
Abdelmotilib H, Maltbie T, Delic V, Liu Z, Hu X, Fraser KB et al (2017) α-Synuclein fibril-induced inclusion spread in rats and mice correlates with dopaminergic neurodegeneration. Neurobiol Dis 105:84–98. https://doi.org/10.1016/j.nbd.2017.05.014
Bousset L, Pieri L, Ruiz-Arlandis G, Gath J, Jensen PH, Habenstein B et al (2013) Structural and functional characterization of two alpha-synuclein strains. Nat Commun 4:2575. https://doi.org/10.1038/ncomms3575
Brás IC, Dominguez-Meijide A, Gerhardt E, Koss D, Lázaro DF, Santos PI et al (2020) Synucleinopathies: where we are and where we need to go. J Neurochem 153:433–454. https://doi.org/10.1111/jnc.14965
Buehler MJ (2006) Nature designs tough collagen: explaining the nanostructure of collagen fibrils. Proc Natl Acad Sci USA 103:12285–12290. https://doi.org/10.1073/pnas.0603216103
Buell AK (2019) The growth of amyloid fibrils: rates and mechanisms. Biochem J 476:2677–2703. https://doi.org/10.1042/bcj20160868
Candelise N, Schmitz M, Llorens F, Villar-Piqué A, Cramm M, Thom T et al (2019) Seeding variability of different alpha synuclein strains in synucleinopathies. Ann Neurol 85:691–703. https://doi.org/10.1002/ana.25446
Chatani E, Lee YH, Yagi H, Yoshimura Y, Naiki H, Goto Y (2009) Ultrasonication-dependent production and breakdown lead to minimum-sized amyloid fibrils. Proc Natl Acad Sci USA 106:11119–11124. https://doi.org/10.1073/pnas.0901422106
Chen GF, Xu TH, Yan Y, Zhou YR, Jiang Y, Melcher K et al (2017) Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin 38:1205–1235. https://doi.org/10.1038/aps.2017.28
Chiti F, Dobson CM (2006) Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75:333–366. https://doi.org/10.1146/annurev.biochem.75.101304.123901
Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT Jr (2000) Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci USA 97:571–576. https://doi.org/10.1073/pnas.97.2.571
Coughlin DG, Hurtig HI, Irwin DJ (2020) Pathological influences on clinical heterogeneity in Lewy body diseases. Mov Disord 35:5–19. https://doi.org/10.1002/mds.27867
De Luca CMG, Elia AE, Portaleone SM, Cazzaniga FA, Rossi M, Bistaffa E et al (2019) Efficient RT-QuIC seeding activity for α-synuclein in olfactory mucosa samples of patients with Parkinson’s disease and multiple system atrophy. Transl Neurodegener 8:24. https://doi.org/10.1186/s40035-019-0164-x
Delic V, Chandra S, Abdelmotilib H, Maltbie T, Wang S, Kem D et al (2018) Sensitivity and specificity of phospho-Ser129 α-synuclein monoclonal antibodies. J Comp Neurol 526:1978–1990. https://doi.org/10.1002/cne.24468
Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, Christie S et al (2016) Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 3:812–818. https://doi.org/10.1002/acn3.338
Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M et al (2004) Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol 55:174–179. https://doi.org/10.1002/ana.10846
Gispert S, Del Turco D, Garrett L, Chen A, Bernard DJ, Hamm-Clement J et al (2003) Transgenic mice expressing mutant A53T human alpha-synuclein show neuronal dysfunction in the absence of aggregate formation. Mol Cell Neurosci 24:419–429. https://doi.org/10.1016/s1044-7431(03)00198-2
Goedert M, Spillantini MG, Del Tredici K, Braak H (2013) 100 years of Lewy pathology. Nat Rev Neurol 9:13–24. https://doi.org/10.1038/nrneurol.2012.242
Groveman BR, Orrù CD, Hughson AG, Raymond LD, Zanusso G, Ghetti B et al (2018) Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol Commun 6:7. https://doi.org/10.1186/s40478-018-0508-2
Guérin G, Wang H, Manners I, Winnik MA (2008) Fragmentation of fiberlike structures: sonication studies of cylindrical block copolymer micelles and behavioral comparisons to biological fibrils. J Am Chem Soc 130:14763–14771. https://doi.org/10.1021/ja805262v
Guerrero-Ferreira R, Taylor NM, Mona D, Ringler P, Lauer ME, Riek R et al (2018) Cryo-EM structure of alpha-synuclein fibrils. Elife. https://doi.org/10.7554/eLife.36402
Hong L, Ko HW, Gwag BJ, Joe E, Lee S, Kim YT et al (1998) The cDNA cloning and ontogeny of mouse alpha-synuclein. NeuroReport 9:1239–1243. https://doi.org/10.1097/00001756-199804200-00051
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC et al (2012) National institute on aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8:1–13. https://doi.org/10.1016/j.jalz.2011.10.007
Kang UJ, Boehme AK, Fairfoul G, Shahnawaz M, Ma TC, Hutten SJ et al (2019) Comparative study of cerebrospinal fluid α-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov Disord 34:536–544. https://doi.org/10.1002/mds.27646
Kim WS, Kågedal K, Halliday GM (2014) Alpha-synuclein biology in Lewy body diseases. Alzheimers Res Ther 6:73. https://doi.org/10.1186/s13195-014-0073-2
Knowles TP, Waudby CA, Devlin GL, Cohen SI, Aguzzi A, Vendruscolo M et al (2009) An analytical solution to the kinetics of breakable filament assembly. Science 326:1533–1537. https://doi.org/10.1126/science.1178250
Konno T, Ross OA, Puschmann A, Dickson DW, Wszolek ZK (2016) Autosomal dominant Parkinson’s disease caused by SNCA duplications. Parkinsonism Relat Disord 22(Suppl 1):S1-6. https://doi.org/10.1016/j.parkreldis.2015.09.007
Kuo YM, Li Z, Jiao Y, Gaborit N, Pani AK, Orrison BM et al (2010) Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum Mol Genet 19:1633–1650. https://doi.org/10.1093/hmg/ddq038
Lau A, So RWL, Lau HHC, Sang JC, Ruiz-Riquelme A, Fleck SC et al (2020) α-Synuclein strains target distinct brain regions and cell types. Nat Neurosci 23:21–31. https://doi.org/10.1038/s41593-019-0541-x
Lavenir I, Passarella D, Masuda-Suzukake M, Curry A, Holton JL, Ghetti B et al (2019) Silver staining (Campbell-Switzer) of neuronal α-synuclein assemblies induced by multiple system atrophy and Parkinson’s disease brain extracts in transgenic mice. Acta Neuropathol Commun 7:148. https://doi.org/10.1186/s40478-019-0804-5
Li B, Ge P, Murray KA, Sheth P, Zhang M, Nair G et al (2018) Cryo-EM of full-length α-synuclein reveals fibril polymorphs with a common structural kernel. Nat Commun 9:3609. https://doi.org/10.1038/s41467-018-05971-2
Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ et al (2012) Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338:949–953. https://doi.org/10.1126/science.1227157
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D et al (2017) Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology 89:88–100. https://doi.org/10.1212/wnl.0000000000004058
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 65:1863–1872. https://doi.org/10.1212/01.wnl.0000187889.17253.b1
McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K et al (1999) Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol 46:860–866. https://doi.org/10.1002/1531-8249(199912)46:6%3c860::aid-ana8%3e3.0.co;2-m
Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, Cooper G et al (2010) Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol 257:359–366. https://doi.org/10.1007/s00415-009-5324-y
Nizynski B, Dzwolak W, Nieznanski K (2017) Amyloidogenesis of Tau protein. Protein Sci 26:2126–2150. https://doi.org/10.1002/pro.3275
Peng C, Gathagan RJ, Covell DJ, Medellin C, Stieber A, Robinson JL et al (2018) Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature 557:558–563. https://doi.org/10.1038/s41586-018-0104-4
Peng Z, Parker AS, Peralta MDR, Ravikumar KM, Cox DL, Toney MD (2017) High tensile strength of engineered β-solenoid fibrils via sonication and pulling. Biophys J 113:1945–1955. https://doi.org/10.1016/j.bpj.2017.09.003
Polinski NK, Volpicelli-Daley LA, Sortwell CE, Luk KC, Cremades N, Gottler LM et al (2018) Best practices for generating and using alpha-synuclein pre-formed fibrils to model Parkinson’s disease in rodents. J Parkinsons Dis 8:303–322. https://doi.org/10.3233/jpd-171248
Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB et al (2015) Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci USA 112:E5308-5317. https://doi.org/10.1073/pnas.1514475112
Rossi M, Candelise N, Baiardi S, Capellari S, Giannini G, Orrù CD et al (2020) Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol 140:49–62. https://doi.org/10.1007/s00401-020-02160-8
Ruf VC, Shi S, Schmidt F, Weckbecker D, Nübling GS, Ködel U et al (2020) Potential sources of interference with the highly sensitive detection and quantification of alpha-synuclein seeds by qRT-QuIC. FEBS Open Bio 10:883–893. https://doi.org/10.1002/2211-5463.12844
Sanderson JB, De S, Jiang H, Rovere M, Jin M, Zaccagnini L et al (2020) Analysis of α-synuclein species enriched from cerebral cortex of humans with sporadic dementia with Lewy bodies. Brain Commun 2:fcaa010. https://doi.org/10.1093/braincomms/fcaa010
Schweighauser M, Shi Y, Tarutani A, Kametani F, Murzin AG, Ghetti B et al (2020) Structures of α-synuclein filaments from multiple system atrophy. Nature 585:464–469. https://doi.org/10.1038/s41586-020-2317-6
Shahnawaz M, Mukherjee A, Pritzkow S, Mendez N, Rabadia P, Liu X et al (2020) Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 578:273–277. https://doi.org/10.1038/s41586-020-1984-7
Smith JF, Knowles TP, Dobson CM, Macphee CE, Welland ME (2006) Characterization of the nanoscale properties of individual amyloid fibrils. Proc Natl Acad Sci USA 103:15806–15811. https://doi.org/10.1073/pnas.0604035103
Strohäker T, Jung BC, Liou SH, Fernandez CO, Riedel D, Becker S et al (2019) Structural heterogeneity of α-synuclein fibrils amplified from patient brain extracts. Nat Commun 10:5535. https://doi.org/10.1038/s41467-019-13564-w
Tarutani A, Arai T, Murayama S, Hisanaga SI, Hasegawa M (2018) Potent prion-like behaviors of pathogenic α-synuclein and evaluation of inactivation methods. Acta Neuropathol Commun 6:29. https://doi.org/10.1186/s40478-018-0532-2
Van der Perren A, Gelders G, Fenyi A, Bousset L, Brito F, Peelaerts W et al (2020) The structural differences between patient-derived α-synuclein strains dictate characteristics of Parkinson’s disease, multiple system atrophy and dementia with Lewy bodies. Acta Neuropathol 139:977–1000. https://doi.org/10.1007/s00401-020-02157-3
van Steenoven I, Majbour NK, Vaikath NN, Berendse HW, van der Flier WM, van de Berg WDJ et al (2018) α-Synuclein species as potential cerebrospinal fluid biomarkers for dementia with lewy bodies. Mov Disord 33:1724–1733. https://doi.org/10.1002/mds.111
Waters CH, Miller CA (1994) Autosomal dominant Lewy body parkinsonism in a four-generation family. Ann Neurol 35:59–64. https://doi.org/10.1002/ana.410350110
Xue WF, Hellewell AL, Gosal WS, Homans SW, Hewitt EW, Radford SE (2009) Fibril fragmentation enhances amyloid cytotoxicity. J Biol Chem 284:34272–34282. https://doi.org/10.1074/jbc.M109.049809
Acknowledgements
These studies have been supported by the National Institutes of Health grants P50 NS108675 and R01 NS064934. The authors acknowledge the generous contributions made by study participants and their families to further research into the causes of DLB and to help find treatments. The authors are indebted to the Duke Kathleen Price Bryan Brain Bank and Biorepository, a component of the Joseph and Kathleen Bryan Alzheimer’s Disease Research Center at Duke University. The authors thank Valentina Krendelchtchikova for her technical assistance, and Sara Miller and Ricardo Vancini for assistance with electron microscopy carried out with the Duke Electron Microscopy Service.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sokratian, A., Ziaee, J., Kelly, K. et al. Heterogeneity in α-synuclein fibril activity correlates to disease phenotypes in Lewy body dementia. Acta Neuropathol 141, 547–564 (2021). https://doi.org/10.1007/s00401-021-02288-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-021-02288-1