Abstract
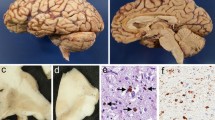
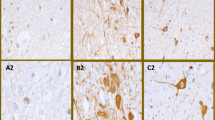
Corticobasal degeneration (CBD) is a disorder affecting cognition and movement due to a progressive neurodegeneration associated with distinctive neuropathologic features, including abnormal phosphorylated tau protein in neurons and glia in cortex, basal ganglia, diencephalon, and brainstem, as well as ballooned neurons and astrocytic plaques. We identified three cases of CBD with olivopontocerebellar atrophy (CBD-OPCA) that did not have α-synuclein-positive glial cytoplasmic inclusions of multiple system atrophy (MSA). Two patients had clinical features suggestive of progressive supranuclear palsy (PSP), and the third case had cerebellar ataxia thought to be due to idiopathic OPCA. Neuropathologic features of CBD-OPCA are compared to typical CBD, as well as MSA and PSP. CBD-OPCA and MSA had marked neuronal loss in pontine nuclei, inferior olivary nucleus, and Purkinje cell layer. Neuronal loss and grumose degeneration in the cerebellar dentate nucleus were comparable in CBD-OPCA and PSP. Image analysis of tau pathology showed greater infratentorial tau burden, especially in pontine base, in CBD-OPCA compared with typical CBD. In addition, CBD-OPCA had TDP-43 immunoreactive neuronal and glial cytoplasmic inclusions and threads throughout the basal ganglia and in olivopontocerebellar system. CBD-OPCA met neuropathologic research diagnostic criteria for CBD and shared tau biochemical characteristics with typical CBD. These results suggest that CBD-OPCA is a distinct clinicopathologic variant of CBD with olivopontocerebellar TDP-43 pathology.







Similar content being viewed by others
References
Ahmed Z, Doherty KM, L Silveira-Moriyama et al (2011) Globular glial tauopathies (GGT) presenting with motor neuron disease or frontotemporal dementia: an emerging group of 4-repeat tauopathies. Acta Neuropathol 122:415–428
Amador-Ortiz C, Lin WL, Ahmed Z et al (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61:435–445
Arai T, Ikeda K, Akiyama H et al (2004) Identification of amino-terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol 55:72–79
Arima K, Ueda K, Sunohara N et al (1998) NACP/alpha-synuclein immunoreactivity in fibrillary components of neuronal and oligodendroglial cytoplasmic inclusions in the pontine nuclei in multiple system atrophy. Acta Neuropathol 96:439–444
Beach TG, White CL, Hamilton RL et al (2008) Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol 116:277–288
Bigio EH, Lipton AM, Yen SH et al (2001) Frontal lobe dementia with novel tauopathy: sporadic multiple system tauopathy with dementia. J Neuropathol Exp Neurol 60:328–341
Buee L, Delacourte A (1999) Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick’s disease. Brain Pathol 9:681–693
DeJesus-Hernandez M, Mackenzie IR, Boeve BF et al (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256
Dickson DW (1999) Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 246(Suppl 2):II6–II15
Dickson DW, Bergeron C, Chin SS et al (2002) Office of rare diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61:935–946
Feany MB, Dickson DW (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146:1388–1396
Fu YJ, Nishihira Y, Kuroda S et al (2010) Sporadic four-repeat tauopathy with frontotemporal lobar degeneration, Parkinsonism, and motor neuron disease: a distinct clinicopathological and biochemical disease entity. Acta Neuropathol 120:21–32
Fujishiro H, Ahn TB, Frigerio R et al (2008) Glial cytoplasmic inclusions in neurologically normal elderly: prodromal multiple system atrophy? Acta Neuropathol 116:269–275
Fujishiro H, Uchikado H, Arai T et al (2009) Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta Neuropathol 117:151–158
Fukutani Y, Nakamura I, Matsubara R, Kobayashi K, Isaki K (1996) Pathology of the cerebellar dentate nucleus in sporadic olivopontocerebellar atrophy: a morphometric investigation. J Neurol Sci 137:103–108
Gibb WR, Luthert PJ, Marsden CD (1989) Corticobasal degeneration. Brain 112(Pt 5):1171–1192
Graham JG, Oppenheimer DR (1969) Orthostatic hypotension and nicotine sensitivity in a case of multiple system atrophy. J Neurol Neurosurg Psychiatry 32:28–34
Greenberg SG, Davies P (1990) A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA 87:5827–5831
Gwinn-Hardy K, Mehta ND, Farrer M et al (2000) Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol (Berl) 99:663–672
Hassan A, Whitwell JL, Boeve BF et al (2010) Symmetric corticobasal degeneration (S-CBD). Parkinsonism Relat Disord 16:208–214
Hu WT, Josephs KA, Knopman SD et al (2008) Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol 116:215–220
Ishizawa K, Lin WL, Tiseo P, Honer WG, Davies P, Dickson DW (2000) A qualitative and quantitative study of grumose degeneration in progressive supranuclear palsy. J Neuropathol Exp Neurol 59:513–524
Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW (2003) Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol 62:389–397
Iwasaki Y, Mori K, Ito M, Mimuro M, Yoshida M (2011) An autopsied case of progressive supranuclear palsy, initially diagnosed as spinocerebellar degeneration with severe olivopontocerebellar involvement. Rinsho Shinkeigaku 51:756–760
Jellinger KA, Lantos PL (2010) Papp-Lantos inclusions and the pathogenesis of multiple system atrophy: an update. Acta Neuropathol 119:657–667
Jicha GA, Petersen RC, Knopman DS et al (2006) Argyrophilic grain disease in demented subjects presenting initially with amnestic mild cognitive impairment. J Neuropathol Exp Neurol 65:602–609
Josephs KA, Petersen RC, Knopman DS et al (2006) Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 66:41–48
Josephs KA, Whitwell JL, Knopman DS et al (2008) Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology 70:1850–1857
Josephs KA, Stroh A, Dugger B, Dickson DW (2009) Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol 118:349–358
Komori T, Arai N, Oda M et al (1998) Astrocytic plaques and tufts of abnormal fibers do not coexist in corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol 96:401–408
Kouri N, Murray ME, A Hassan et al (2011) Neuropathological features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome. Brain 134:3264–3275
Kouri N, Whitwell JL, Josephs KA, Rademakers R, Dickson DW (2011) Corticobasal degeneration: a pathologically distinct 4R tauopathy. Nat Rev Neurol 7:263–272
Kovacs GG, Majtenyi K, Spina S et al (2008) White matter tauopathy with globular glial inclusions: a distinct sporadic frontotemporal lobar degeneration. J Neuropathol Exp Neurol 67:963–975
Mizusawa H, Yen SH, Hirano A, Llena JF (1989) Pathology of the dentate nucleus in progressive supranuclear palsy: a histological, immunohistochemical and ultrastructural study. Acta Neuropathol 78:419–428
Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW (2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10:785–796
Papp MI, Kahn JE, Lantos PL (1989) Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci 94:79–100
Rebeiz JJ, Kolodny EH, Richardson EP Jr (1967) Corticodentatonigral degeneration with neuronal achromasia: a progressive disorder of late adult life. Trans Am Neurol Assoc 92:23–26
Togo T, Cookson N, Dickson DW (2002) Argyrophilic grain disease: neuropathology, frequency in a dementia brain bank and lack of relationship with apolipoprotein E. Brain Pathol 12:45–52
Togo T, Sahara N, Yen SH et al (2002) Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol 61:547–556
Tolnay M, Spillantini MG, Goedert M, Ulrich J, Langui D, Probst A (1997) Argyrophilic grain disease: widespread hyperphosphorylation of tau protein in limbic neurons. Acta Neuropathol 93:477–484
Uryu K, Nakashima-Yasuda H, Forman MS et al (2008) Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 67:555–564
Williams DR, Lees AJ, Wherrett JR, Steele JC (2008) J. Clifford Richardson and 50 years of progressive supranuclear palsy. Neurology 70:566–573
Yokota O, Davidson Y, Bigio EH et al (2010) Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol 120:55–66
Acknowledgments
The authors thank Virginia Philips, Linda Rousseau, and Monica Castanedes-Casey for their expert technical assistance, Dr. Peter Davies at The Feinstein Institute for Medical Research, Manhasset, NY, for the gifts of CP13 and PHF-1 antibodies, and Dr. Rosa Rademakers for assisting with the genetic studies. Two of the CBD-OPCA cases and most of the PSP and CBD cases in this study were submitted to the CurePSP brain bank at Mayo Clinic in Jacksonville, FL; one was originally submitted to Department of Pathology and Laboratory Medicine diagnostic evaluation at Mayo Clinic in Rochester, MN. The studies would not have been possible without generous donations of family members and their contributions in this endeavor are greatly appreciated. This study was supported by NIH grants: P50-AG016574, P50–NS072187, CurePSP | Society for Progressive Supranuclear Palsy, The Robert E. Jacoby Professorship for Alzheimer’s Research, and Mayo Foundation for Education and Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
N. Kouri and K. Oshima contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kouri, N., Oshima, K., Takahashi, M. et al. Corticobasal degeneration with olivopontocerebellar atrophy and TDP-43 pathology: an unusual clinicopathologic variant of CBD. Acta Neuropathol 125, 741–752 (2013). https://doi.org/10.1007/s00401-013-1087-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-013-1087-8