Abstract
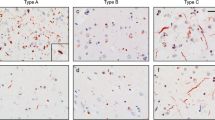
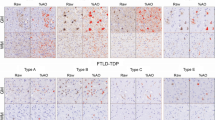
Frontotemporal lobar degeneration (FTLD) can be classified as tau-positive (FTLD-tau) and tau-negative FTLD. The most common form of tau-negative FTLD is associated with neuronal inclusions that are composed of TAR DNA-binding protein 43 (TDP-43) (FTLD-TDP). Recent evidence suggests that FTLD-TDP can be further subdivided into at least three major histologic variants based on patterns of TDP-43 immunoreactive neuronal cytoplasmic inclusions (NCI) and dystrophic neurites (DN) in neocortex and hippocampus. The aim of this study was to extend the histologic analysis to other brain regions and to determine if there were distinct clinical and pathologic characteristics of the FTLD-TDP subtypes. Thirty-nine FTLD-TDP cases were analyzed (Mackenzie type 1 n = 24, Mackenzie type 2 n = 9, Mackenzie type 3 n = 6). There was a highly significant association between clinical syndrome and FTLD-TDP subtype, with progressive non-fluent aphasia associated with type 1, semantic dementia with type 2, and behavioral variant frontotemporal dementia with types 1, 2 and 3. Semi-quantitative analysis of NCI and DN demonstrated different patterns of involvement in cortical, subcortical and brainstem areas that were characteristic for each of the three types of FTLD-TDP. Type 1 had a mixture of NCI and DN, as well as intranuclear inclusions in most cases and TDP-43 pathology at all levels of the neuraxis, but less in brainstem than supratentorial structures. Type 2 cases were characterized by predominance of long, thick DN in the cortex, as well as numerous NCI in hippocampus, amygdala and basal ganglia, but virtually no NCI and only sparse DN in diencephalon and brainstem. Type 3 had a paucity of DN at all levels of the neuraxis and significantly more NCI in the hypoglossal nucleus than the other types. These findings extend previously described clinicopathological associations of FTLD-TDP subtypes and support the notion that FTLD-TDP subtypes may be distinct clinicopathologic disorders.








Similar content being viewed by others
References
Baker M, Mackenzie IR, Pickering-Brown SM et al (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442:916–919. doi:10.1038/nature05016
Beach TG, White CL, Hamilton RL et al (2008) Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol 116:277–288. doi:10.1007/s00401-008-0409-8
Cairns NJ, Bigio EH, Mackenzie IR et al (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22. doi:10.1007/s00401-007-0237-2
Cairns NJ, Grossman M, Arnold SE et al (2004) Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology 63:1376–1384
Dachsel JC, Ross OA, Mata IF et al (2007) Lrrk2 G2019S substitution in frontotemporal lobar degeneration with ubiquitin-immunoreactive neuronal inclusions. Acta Neuropathol 113:601–606. doi:10.1007/s00401-006-0178-1
Davidson Y, Kelley T, Mackenzie IR et al (2007) Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol 113:521–533. doi:10.1007/s00401-006-0189-y
Dickson DW (2005) Required techniques and useful molecular markers in the neuropathologic diagnosis of neurodegenerative diseases. Acta Neuropathol 109:14–24. doi:10.1007/s00401-004-0950-z
Dickson DW, Josephs KA, Amador-Ortiz C (2007) TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol 114:71–79. doi:10.1007/s00401-007-0234-5
Forman MS, Farmer J, Johnson JK et al (2006) Frontotemporal dementia: clinicopathological correlations. Ann Neurol 59:952–962. doi:10.1002/ana.20873
Grossman M, Wood EM, Moore P et al (2007) TDP-43 pathologic lesions and clinical phenotype in frontotemporal lobar degeneration with ubiquitin-positive inclusions. Arch Neurol 64:1449–1454. doi:10.1001/archneur.64.10.1449
Gwinn-Hardy K, Mehta ND, Farrer M et al (2000) Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol 99:663–672. doi:10.1007/s004010051177
Hatanpaa KJ, Bigio EH, Cairns NJ et al (2008) TAR DNA-binding protein 43 immunohistochemistry reveals extensive neuritic pathology in FTLD-U: a midwest-southwest consortium for FTLD study. J Neuropathol Exp Neurol 67:271–279. doi:10.1097/NEN.0b013e31816a12a6
Hodges JR, Davies RR, Xuereb JH et al (2004) Clinicopathological correlates in frontotemporal dementia. Ann Neurol 56:399–406. doi:10.1002/ana.20203
Josephs KA, Ahmed Z, Katsuse O et al (2007) Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol 66:142–151. doi:10.1097/nen.0b013e31803020cf
Josephs KA, Dickson DW (2007) Hippocampal sclerosis in tau-negative frontotemporal lobar degeneration. Neurobiol Aging 28:1718–1722. doi:10.1016/j.neurobiolaging.2006.07.010
Josephs KA, Dickson DW (2007) Frontotemporal lobar degeneration with upper motor neuron disease/primary lateral sclerosis. Neurology 69:1800–1801. doi:10.1212/01.wnl.0000277270.99272.7e
Josephs KA, Holton JL, Rossor MN et al (2003) Neurofilament inclusion body disease: a new proteinopathy? Brain 126:2291–2303. doi:10.1093/brain/awg231
Josephs KA, Holton JL, Rossor MN et al (2004) Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol 30:369–373. doi:10.1111/j.1365-2990.2003.00545.x
Josephs KA, Knopman DS, Whitwell JL et al (2005) Survival in two variants of tau-negative frontotemporal lobar degeneration: FTLD-U vs FTLD-MND. Neurology 65:645–647. doi:10.1212/01.wnl.0000173178.67986.7f
Josephs KA, Lin WL, Ahmed Z, Stroh DA, Graff-Radford NR, Dickson DW (2008) Frontotemporal lobar degeneration with ubiquitin-positive, but TDP-43-negative inclusions. Acta Neuropathol 116:159–167. doi:10.1007/s00401-008-0397-8
Josephs KA, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Dickson DW (2006) Clinically undetected motor neuron disease in pathologically proven frontotemporal lobar degeneration with motor neuron disease. Arch Neurol 63:506–512. doi:10.1001/archneur.63.4.506
Josephs KA, Petersen RC, Knopman DS et al (2006) Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 66:41–48. doi:10.1212/01.wnl.0000191307.69661.c3
Josephs KA, Uchikado H, McComb RD et al (2005) Extending the clinicopathological spectrum of neurofilament inclusion disease. Acta Neuropathol 109:427–432. doi:10.1007/s00401-004-0974-4
Katsuse O, Dickson DW (2005) Ubiquitin immunohistochemistry of frontotemporal lobar degeneration differentiates cases with and without motor neuron disease. Alzheimer Dis Assoc Disord 19(Suppl 1):S37–S43. doi:10.1097/01.wad.0000183889.61421.a8
Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG (2005) The evolution and pathology of frontotemporal dementia. Brain 128:1996–2005. doi:10.1093/brain/awh598
Mackenzie IR (2007) The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta Neuropathol 114:49–54. doi:10.1007/s00401-007-0223-8
Mackenzie IR, Baborie A, Pickering-Brown S et al (2006) Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol 112:539–549. doi:10.1007/s00401-006-0138-9
Mackenzie IR, Neumann M, Bigio EH et al (2009) Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol 117:15–18. doi:10.1007/s00401-008-0460-5
McCluskey LF, Elman LB, Martinez-Lage M et al (2009) Amyotrophic lateral sclerosis-plus syndrome with TAR DNA-binding protein-43 pathology. Arch Neurol 66:121–124. doi:10.1001/archneur.66.1.121
McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ (2001) Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol 58:1803–1809. doi:10.1001/archneur.58.11.1803
Neary D, Snowden JS, Gustafson L et al (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133. doi:10.1126/science.1134108
Pikkarainen M, Hartikainen P, Alafuzoff I (2008) Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions visualized with ubiquitin-binding protein p62 immunohistochemistry. J Neuropathol Exp Neurol 67:280–298. doi:10.1097/NEN.0b013e31816a1da2
Sampathu DM, Neumann M, Kwong LK et al (2006) Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol 169:1343–1352. doi:10.2353/ajpath.2006.060438
Snowden J, Neary D, Mann D (2007) Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol 114:31–38. doi:10.1007/s00401-007-0236-3
Thompson SA, Patterson K, Hodges JR (2003) Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology 61:1196–1203
Togo T, Cookson N, Dickson DW (2002) Argyrophilic grain disease: neuropathology, frequency in a dementia brain bank and lack of relationship with apolipoprotein E. Brain Pathol 12:45–52
Uchikado H, Li A, Lin WL, Dickson DW (2006) Heterogeneous inclusions in neurofilament inclusion disease. Neuropathology 26:417–421. doi:10.1111/j.1440-1789.2006.00709.x
Acknowledgments
K. A. J. is supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12/NICHHD)-HD49078. D. W. D. is supported by NIH grants: P50AG025711, P01AG017216, P01AG03949 and P50NS40256. Some of the cases used in this study were derived from the State of Florida Alzheimer Disease Initiative brain bank, which is funded by the State of Florida Department of Elder Affairs, while others came from the Society for Progressive Supranuclear Palsy brain bank. We acknowledge the contribution of the many clinicians in the Mayo Clinic and referring centers for their evaluations of the patients in this study and the families who gave permission for postmortem studies to elucidate the cause, treatment and prevention of these disorders. We acknowledge the histological assistance of Virginia Phillips, Linda Rousseau and Monica Casey-Castanedes. Genetic analyses were performed by Matthew Baker; Rosa Rademakers, Justus Dachsel and Matthew Farrer on some of the cases, as part of ongoing studies of FTLD and parkinsonism.
Conflict of interest statement
The authors do not have any disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Josephs, K.A., Stroh, A., Dugger, B. et al. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol 118, 349–358 (2009). https://doi.org/10.1007/s00401-009-0547-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-009-0547-7