Abstract
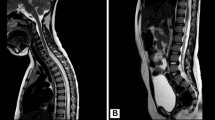
Rare, generally pediatric oligodendroglioma-like neoplasms with extensive leptomeningeal dissemination have been interpreted variably as glial, oligodendroglial or glioneuronal. The clinicopathologic features have not been fully characterized. We studied 36 patients, 12 females and 24 males with a median age of 5 years (range 5 months–46 years). MRI demonstrated leptomeningeal enhancement, frequently with cystic or nodular T2 hyperintense lesions within the spinal cord/brain along the subpial surface. A discrete intraparenchymal lesion, usually in the spinal cord, was found in 25 (of 31) (81 %). Tumors contained oligodendroglioma-like cells with low-mitotic activity (median 0 per 10 high power fields, range 0–4), and rare ganglion/ganglioid cells in 6 cases (17 %). Tumors were mostly low-grade, with anaplastic progression in 8 (22 %). Immunohistochemistry demonstrated strong reactivity for OLIG2 (7 of 9) (78 %), and moderate/strong S100 (11 of 12) (92 %), GFAP (12 of 31) (39 %) and synaptophysin (19 of 27) (70 %). NeuN, EMA, and mutant IDH1 (R132H) protein were negative. Median MIB1 labeling index was 1.5 % (range <1–30 %). FISH (n = 13) or SNP array (n = 2) demonstrated 1p loss/intact 19q in 8 (53 %), 1p19q co-deletion in 3 (20 %), and no 1p or 19q loss in 4 (27 %). Clinical follow-up (n = 24) generally showed periods of stability or slow progression, but a subset of tumors progressed to anaplasia and behaved more aggressively. Nine patients (38 %) died 3 months–21 years after diagnosis (median total follow-up 5 years). We report a series of a neoplasm with distinct clinicopathologic and molecular features. Although most progress slowly, a significant fraction develop aggressive features.










Similar content being viewed by others
References
Agamanolis DP, Katsetos CD, Klonk CJ, Bartkowski HM, Ganapathy S, Staugaitis SM, Kuerbitz SJ, Patton DF, Talaizadeh A, Cohen BH (2012) An unusual form of superficially disseminated glioma in children: report of 3 cases. J Child Neurol 27:727–733
Armao DM, Stone J, Castillo M, Mitchell KM, Bouldin TW, Suzuki K (2000) Diffuse leptomeningeal oligodendrogliomatosis: radiologic/pathologic correlation. AJNR 21:1122–1126
Beck DJ, Russell DS (1942) Oligodendrogliomatosis of the cerebrospinal pathway. Brain 65:352–372
Bettegowda C, Agrawal N, Jiao Y, Sausen M, Wood LD, Hruban RH, Rodriguez FJ, Cahill DP, McLendon R, Riggins G, Velculescu VE, Oba-Shinjo SM, Marie SK, Vogelstein B, Bigner D, Yan H, Papadopoulos N, Kinzler KW (2011) Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science 333:1453–1455
Bourne TD, Mandell JW, Matsumoto JA, Jane JA Jr, Lopes MB (2006) Primary disseminated leptomeningeal oligodendroglioma with 1p deletion. Case report. J Neurosurg 105:465–469
Capper D, Reuss D, Schittenhelm J, Hartmann C, Bremer J, Sahm F, Harter PN, Jeibmann A, von Deimling A (2011) Mutation-specific IDH1 antibody differentiates oligodendrogliomas and oligoastrocytomas from other brain tumors with oligodendroglioma-like morphology. Acta Neuropathol 121:241–252
Chen R, Macdonald DR, Ramsay DA (1995) Primary diffuse leptomeningeal oligodendroglioma. Case report. J Neurosurg 83:724–728
Civitello LA, Packer RJ, Rorke LB, Siegel K, Sutton LN, Schut L (1988) Leptomeningeal dissemination of low-grade gliomas in childhood. Neurology 38:562–566
Daum S, Foncin JF, Nicolaidis S, Oeconomos D (1974) Diffuse gliomatosis of the leptomeninges and tuber oligodendrioma. Rev Neurol (Paris) 130:314–320
Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, Woo S, Wheeler G, Ahern V, Krasin MJ, Fouladi M, Broniscer A, Krance R, Hale GA, Stewart CF, Dauser R, Sanford RA, Fuller C, Lau C, Boyett JM, Wallace D, Gilbertson RJ (2006) Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol 7:813–820
Gardiman MP, Fassan M, Orvieto E, D’Avella D, Denaro L, Calderone M, Severino M, Scarsello G, Viscardi E, Perilongo G (2010) Diffuse leptomeningeal glioneuronal tumors: a new entity? Brain Pathol 20:361–366
Gilmer-Hill HS, Ellis WG, Imbesi SG, Boggan JE (2000) Spinal oligodendroglioma with gliomatosis in a child. Case report. J Neurosurg 92:109–113
Griffin CA, Burger P, Morsberger L, Yonescu R, Swierczynski S, Weingart JD, Murphy KM (2006) Identification of der(1;19)(q10;p10) in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss. J Neuropathol Exp Neurol 65:988–994
Guppy KH, Akins PT, Moes GS, Prados MD (2009) Spinal cord oligodendroglioma with 1p and 19q deletions presenting with cerebral oligodendrogliomatosis. J Neurosurg Spine 10:557–563
Harada S, Henderson LB, Eshleman JR, Gocke CD, Burger P, Griffin CA, Batista DA (2011) Genomic changes in gliomas detected using single nucleotide polymorphism array in formalin-fixed, paraffin-embedded tissue: superior results compared with microsatellite analysis. J Mol Diagn 13:541–548
Hervey-Jumper SL, Jumper M, Blaivas M, Parmar HA, Robertson PL, Maher CO (2010) Primary diffuse leptomeningeal oligodendroglioma. Pediatr Neurosurg 46:326–328
Horbinski C, Miller CR, Perry A (2011) Gone FISHing: clinical lessons learned in brain tumor molecular diagnostics over the last decade. Brain Pathol 21:57–73
Huang T, Zimmerman RA, Perilongo G, Kaufman BA, Holden KR, Carollo C, Chong WK (2001) An unusual cystic appearance of disseminated low-grade gliomas. Neuroradiology 43:868–874
Jenkins RB, Blair H, Ballman KV, Giannini C, Arusell RM, Law M, Flynn H, Passe S, Felten S, Brown PD, Shaw EG, Buckner JC (2006) A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res 66:9852–9861
Jentoft M, Giannini C, Rossi S, Mota R, Jenkins RB, Rodriguez FJ (2011) Oligodendroglial tumors with marked desmoplasia: clinicopathologic and molecular features of 7 cases. Am J Surg Pathol 35:845–852
Jones DT, Mulholland SA, Pearson DM, Malley DS, Openshaw SW, Lambert SR, Liu L, Backlund LM, Ichimura K, Collins VP (2011) Adult grade II diffuse astrocytomas are genetically distinct from and more aggressive than their paediatric counterparts. Acta Neuropathol 121:753–761
Korein J, Feigin I, Shapiro MF (1957) Oligodendrogliomatosis with intracranial hypertension. Neurology 7:589–594
Kreiger PA, Okada Y, Simon S, Rorke LB, Louis DN, Golden JA (2005) Losses of chromosomes 1p and 19q are rare in pediatric oligodendrogliomas. Acta Neuropathol 109:387–392
Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL, Louis DN, Stiles CD, Rowitch DH (2004) The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol 63:499–509
Lu QR, Park JK, Noll E, Chan JA, Alberta J, Yuk D, Alzamora MG, Louis DN, Stiles CD, Rowitch DH, Black PM (2001) Oligodendrocyte lineage genes (OLIG) as molecular markers for human glial brain tumors. Proc Natl Acad Sci USA 98:10851–10856
Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH (2002) Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109:75–86
Mathews MS, Pare LS, Kuo JV, Kim RC (2009) Primary leptomeningeal oligodendrogliomatosis. J Neurooncol 94:275–278
Michotte A, Chaskis C, Sadones J, Veld PI, Neyns B (2009) Primary leptomeningeal anaplastic oligodendroglioma with a 1p36-19q13 deletion: report of a unique case successfully treated with Temozolomide. J Neurol Sci 287:267–270
Mittelbronn M, Wolff M, Bultmann E, Nagele T, Capper D, Beck R, Meyermann R, Beschorner R (2005) Disseminating anaplastic brainstem oligodendroglioma associated with allelic loss in the tumor suppressor candidate region D19S246 of chromosome 19 mimicking an inflammatory central nervous system disease in a 9-year-old boy. Hum Pathol 36:854–857
Ng HK, Poon WS (1999) Diffuse leptomeningeal gliomatosis with oligodendroglioma. Pathology 31:59–63
Perry A, Scheithauer BW, Macaulay RJ, Raffel C, Roth KA, Kros JM (2002) Oligodendrogliomas with neurocytic differentiation. A report of 4 cases with diagnostic and histogenetic implications. J Neuropathol Exp Neurol 61:947–955
Perry A, Burton SS, Fuller GN, Robinson CA, Palmer CA, Resch L, Bigio EH, Gujrati M, Rosenblum MK (2010) Oligodendroglial neoplasms with ganglioglioma-like maturation: a diagnostic pitfall. Acta Neuropathol 120:237–252
Preusser M, Budka H, Rossler K, Hainfellner JA (2007) OLIG2 is a useful immunohistochemical marker in differential diagnosis of clear cell primary CNS neoplasms. Histopathology 50:365–370
Psarros TG, Swift D, Mulne AF, Burns DK (2005) Neurocytoma-like neoplasm of the thoracic spine in a 15-month-old child presenting with diffuse leptomeningeal dissemination and communicating hydrocephalus. Case report. J Neurosurg 103:184–190
Raghavan R, Balani J, Perry A, Margraf L, Vono MB, Cai DX, Wyatt RE, Rushing EJ, Bowers DC, Hynan LS, White CL 3rd (2003) Pediatric oligodendrogliomas: a study of molecular alterations on 1p and 19q using fluorescence in situ hybridization. J Neuropathol Exp Neurol 62:530–537
Reynolds RM, Boswell E, Hulette CM, Cummings TJ, Haglund MM, Cumm Ings TJ (2011) Sudden death from diffuse leptomeningeal oligodendrogliomatosis. J Neurosurg Spine 15:625–629
Rodriguez FJ, Mota RA, Scheithauer BW, Giannini C, Blair H, New KC, Wu KJ, Dickson DW, Jenkins RB (2009) Interphase cytogenetics for 1p19q and t(1;19)(q10;p10) may distinguish prognostically relevant subgroups in extraventricular neurocytoma. Brain Pathol 19:623–629
Rogers LR, Estes ML, Rosenbloom SA, Harrold L (1995) Primary leptomeningeal oligodendroglioma: case report. Neurosurgery 36:166–168 discussion 169
Roldan G, Scott J, George D, Parney I, Easaw J, Cairncross G, Forsyth P, Yan E (2008) Leptomeningeal disease from oligodendroglioma: clinical and molecular analysis. Can J Neurol Sci 35:204–209
Rossi S, Rodriguez FJ, Mota RA, Dei Tos AP, Di Paola F, Bendini M, Agostini S, Longatti P, Jenkins RB, Giannini C (2009) Primary leptomeningeal oligodendroglioma with documented progression to anaplasia and t(1;19)(q10;p10) in a child. Acta Neuropathol 118:575–577
Senaratna S, Hanieh A, Manson J, Toogood I (2001) Multiple cystic brain lesions in a patient with pilocytic astrocytoma. J Clin Neurosci 8:363–366
Stodberg T, Deniz Y, Esteitie N, Jacobsson B, Mousavi-Jazi M, Dahl H, Zweygberg Wirgart B, Grillner L, Linde A (2002) A case of diffuse leptomeningeal oligodendrogliomatosis associated with HHV-6 variant A. Neuropediatrics 33:266–270
Ushida T, Sonobe H, Mizobuchi H, Toda M, Tani T, Yamamoto H (1998) Oligodendroglioma of the “widespread” type in the spinal cord. Childs Nerv Syst 14:751–755
Utsuki S, Oka H, Kijima C, Yasui Y, Fujii K, Kawano N (2012) Pilocytic astrocytoma with abundant oligodendroglioma-like component. Brain Tumor Pathol 29:103–106
Vasiljevic A, Francois P, Loundou A, Fevre-Montange M, Jouvet A, Roche PH, Figarella-Branger D (2012) Prognostic factors in central neurocytomas: a multicenter study of 71 cases. Am J Surg Pathol 36:220–227
Vyberg M, Ulhoi BP, Teglbjaerg PS (2007) Neuronal features of oligodendrogliomas—an ultrastructural and immunohistochemical study. Histopathology 50:887–896
Wakabayashi K, Shimura T, Mizutani N, Koide A, Yamagiwa O, Mori F, Nishiyama K, Tanaka R, Takahashi H (2002) Primary intracranial solitary leptomeningeal glioma: a report of 3 cases. Clin Neuropathol 21:206–213
Yamamoto T, Komori T, Shibata N, Toyoda C, Kobayashi M (1996) Multifocal neurocytoma/gangliocytoma with extensive leptomeningeal dissemination in the brain and spinal cord. Am J Surg Pathol 20:363–370
Yip S, Butterfield YS, Morozova O, Chittaranjan S, Blough MD, An J, Birol I, Chesnelong C, Chiu R, Chuah E, Corbett R, Docking R, Firme M, Hirst M, Jackman S, Karsan A, Li H, Louis DN, Maslova A, Moore R, Moradian A, Mungall KL, Perizzolo M, Qian J, Roldan G, Smith EE, Tamura-Wells J, Thiessen N, Varhol R, Weiss S, Wu W, Young S, Zhao Y, Mungall AJ, Jones SJ, Morin GB, Chan JA, Cairncross JG, Marra MA (2012) Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J Pathol 226:7–16
Zentner J, Anagnostopoulos J, Gilsbach J (1988) Solitary intracranial extracerebral glioma. Neurochirurgia (Stuttg) 31:101–103
Zhou Q, Anderson DJ (2002) The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109:61–73
Acknowledgments
The work was supported in part by the Pediatric Low Grade Astrocytoma (PLGA) foundation and the Pilocytic/Pilomyxoid Astrocytoma (PA/PMA) fund. The authors also thank the pathologists who kindly contributed cases.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rodriguez, F.J., Perry, A., Rosenblum, M.K. et al. Disseminated oligodendroglial-like leptomeningeal tumor of childhood: a distinctive clinicopathologic entity. Acta Neuropathol 124, 627–641 (2012). https://doi.org/10.1007/s00401-012-1037-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-012-1037-x