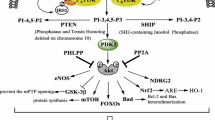
Abstract
The N-myc downstream-regulated gene 2 (NDRG2) is involved in cell apoptosis and survival. Although reported to be highly expressed in the cardiac tissue, the biological function of NDRG2 in the heart remains to be established. Insulin exerts protective effects against myocardial ischemia/reperfusion (I/R) injury through the PI3K/Akt pathway. Here, we examined the changes in phosphorylation of NDRG2, a novel substrate and phosphoprotein of Akt, in insulin-induced protection against myocardial I/R. Rat hearts were subjected to 30 min regional ischemia followed by reperfusion with or without insulin at the onset of reperfusion. Reperfusion with insulin inhibited myocardial apoptosis and reduced infarct size, as well as significantly up-regulated myocardial Akt and NDRG2 phosphorylation levels compared with the I/R group. These effects of insulin were blocked by pretreatment with the PI3K inhibitor wortmannin or Akt inhibitor. To further ascertain the role of NDRG2 in insulin-induced cardioprotection, cardiomyocytes were transduced with a lentivirus encoding shRNA targeting NDRG2 (loss-of-function), which rendered the cells more susceptible to I/R injury and significantly blunted the anti-apoptotic effect of insulin. Moreover, the NDRG2 shRNA lentivirus was tested in vivo, and NDRG2 knockdown aggravated myocardial I/R injury and attenuated the insulin-mediated cardioprotection against I/R injury. Taken together, these results suggest a novel role of PI3K/Akt/NDRG2 signaling in the cardioprotective effect of insulin.








Similar content being viewed by others
References
Boulkroun S, Fay M, Zennaro MC, Escoubet B, Jaisser F, Blot-Chabaud M, Farman N, Courtois-Coutry N (2002) Characterization of rat NDRG2 (N-Myc downstream regulated gene 2), a novel early mineralocorticoid-specific induced gene. J Biol Chem 277:31506–31515. doi:10.1074/jbc.M200272200
Boulkroun S, Le Moellic C, Blot-Chabaud M, Farman N, Courtois-Coutry N (2005) Expression of androgen receptor and androgen regulation of NDRG2 in the rat renal collecting duct. Pflugers Arch 451:388–394. doi:0.1007/s00424-005-1410-x
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868. doi:10.1016/S0092-8674(00)80595-4
Burchfield JG, Lennard AJ, Narasimhan S, Hughes WE, Wasinger VC, Corthals GL, Okuda T, Kondoh H, Biden TJ, Schmitz-Peiffer C (2004) Akt mediates insulin-stimulated phosphorylation of Ndrg2: evidence for cross-talk with protein kinase C theta. J Biol Chem 279:18623–18632. doi:10.1074/jbc.M401504200
Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318–1321. doi:10.1126/science.282.5392.1318
Choi SC, Kim KD, Kim JT, Kim JW, Yoon DY, Choe YK, Chang YS, Paik SG, Lim JS (2003) Expression and regulation of NDRG2 (N-myc downstream regulated gene 2) during the differentiation of dendritic cells. FEBS Lett 553:413–418. doi:S0014579303010305
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241. doi:10.1016/S0092-8674(00)80405-5
Del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G (1997) Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278:687–689. doi:10.1126/science.278.5338.687
Deng Y, Yao L, Chau L, Ng SS, Peng Y, Liu X, Au WS, Wang J, Li F, Ji S, Han H, Nie X, Li Q, Kung HF, Leung SY, Lin MC (2003) N-Myc downstream-regulated gene 2 (NDRG2) inhibits glioblastoma cell proliferation. Int J Cancer 106:342–347. doi:10.1002/ijc.11228
Esumi K, Nishida M, Shaw D, Smith TW, Marsh JD (1991) NADH measurements in adult rat myocytes during simulated ischemia. Am J Physiol 260:H1743–H1752
Foletta VC, Prior MJ, Stupka N, Carey K, Segal DH, Jones S, Swinton C, Martin S, Cameron-Smith D, Walder KR (2009) NDRG2, a novel regulator of myoblast proliferation, is regulated by anabolic and catabolic factors. J Physiol 587:1619–1634. doi:jphysiol.2008.167882
Fuglesteg BN, Suleman N, Tiron C, Kanhema T, Lacerda L, Andreasen TV, Sack MN, Jonassen AK, Mjos OD, Opie LH, Lecour S (2008) Signal transducer and activator of transcription 3 is involved in the cardioprotective signalling pathway activated by insulin therapy at reperfusion. Basic Res Cardiol 103:444–453. doi:10.1007/s00395-008-0728-x
Gao F, Gao E, Yue TL, Ohlstein EH, Lopez BL, Christopher TA, Ma XL (2002) Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation 105:1497–1502. doi:10.1161/01.CIR.0000012529.00367.0F
Ghaboura N, Tamareille S, Ducluzeau PH, Grimaud L, Loufrani L, Croue A, Tourmen Y, Henrion D, Furber A, Prunier F (2011) Diabetes mellitus abrogates erythropoietin-induced cardioprotection against ischemic-reperfusion injury by alteration of the RISK/GSK-3beta signaling. Basic Res Cardiol 106:147–162. doi:10.1007/s00395-010-0130-3
Grace PA (1994) Ischaemia-reperfusion injury. Br J Surg 81:637–647
Grill CJ, Sivaprasad U, Cohick WS (2002) Constitutive expression of IGF-binding protein-3 by mammary epithelial cells alters signaling through Akt and p70S6 kinase. J Mol Endocrinol 29:153–162. doi:10.1677/jme.0.0290153
Hausenloy DJ, Yellon DM (2004) New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res 61:448–460. doi:10.1016/j.cardiores.2003.09.024
Heusch G (2013) Cardioprotection: chances and challenges of its translation to the clinic. Lancet 381:166–175. doi:10.1016/S0140-6736(12)60916-7
Heusch G, Boengler K, Schulz R (2008) Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation 118:1915–1919. doi:10.1161/CIRCULATIONAHA.108.805242
Heusch G, Musiolik J, Gedik N, Skyschally A (2011) Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res 109:1302–1308. doi:10.1161/CIRCRESAHA.111.255604
Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J (2011) Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol 106:1173–1191. doi:10.1007/s00395-011-0222-8
Klippel A, Kavanaugh WM, Pot D, Williams LT (1997) A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol 17:338–344
Kohn AD, Takeuchi F, Roth RA (1996) Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J Biol Chem 271:21920–21926
Lawlor MA, Alessi DR (2001) PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 114:2903–2910
Levkau B, Schafers M, Wohlschlaeger J, von Wnuck Lipinski K, Keul P, Hermann S, Kawaguchi N, Kirchhof P, Fabritz L, Stypmann J, Stegger L, Flogel U, Schrader J, Fischer JW, Hsieh P, Ou YL, Mehrhof F, Tiemann K, Ghanem A, Matus M, Neumann J, Heusch G, Schmid KW, Conway EM, Baba HA (2008) Survivin determines cardiac function by controlling total cardiomyocyte number. Circulation 117:1583–1593. doi:10.1161/CIRCULATIONAHA.107.734160
Li J, Wu F, Zhang H, Fu F, Ji L, Dong L, Li Q, Liu W, Zhang Y, Lv A, Wang H, Ren J, Gao F (2009) Insulin inhibits leukocyte-endothelium adherence via an Akt-NO-dependent mechanism in myocardial ischemia/reperfusion. J Mol Cell Cardiol 47:512–519. doi:10.1016/j.yjmcc.2009.07.010
Li J, Zhang H, Wu F, Nan Y, Ma H, Guo W, Wang H, Ren J, Das UN, Gao F (2008) Insulin inhibits tumor necrosis factor-alpha induction in myocardial ischemia/reperfusion: role of Akt and endothelial nitric oxide synthase phosphorylation. Crit Care Med 36:1551–1558. doi:10.1097/CCM.0b013e3181782335
Li QX, Xiong ZY, Hu BP, Tian ZJ, Zhang HF, Gou WY, Wang HC, Gao F, Zhang QJ (2009) Aging-associated insulin resistance predisposes to hypertension and its reversal by exercise: the role of vascular vasorelaxation to insulin. Basic Res Cardiol 104:269–284. doi:10.1007/s00395-008-0754-8
Li Y, Yang J, Li S, Zhang J, Zheng J, Hou W, Zhao H, Guo Y, Liu X, Dou K, Situ Z, Yao L (2011) N-myc downstream-regulated gene 2, a novel estrogen-targeted gene, is involved in the regulation of Na+/K+-ATPase. J Biol Chem 286:32289–32299. doi:10.1074/jbc.M111.247825
Liu J, Zhang J, Wang X, Li Y, Chen Y, Li K, Yao L, Guo G (2010) HIF-1 and NDRG2 contribute to hypoxia-induced radioresistance of cervical cancer Hela cells. Exp Cell Res 316:1985–1993. doi:10.1016/j.yexcr.2010.02.028
Liu S, Yang P, Kang H, Lu L, Zhang Y, Pan J, Rui YC (2010) NDRG2 induced by oxidized LDL in macrophages antagonizes growth factor productions via selectively inhibiting ERK activation. Biochim Biophys Acta 1801:106–113. doi:10.1016/j.bbalip.2009.09.022
Lusis EA, Watson MA, Chicoine MR, Lyman M, Roerig P, Reifenberger G, Gutmann DH, Perry A (2005) Integrative genomic analysis identifies NDRG2 as a candidate tumor suppressor gene frequently inactivated in clinically aggressive meningioma. Cancer Res 65:7121–7126. doi:10.1158/0008-5472.CAN-05-0043
Ma J, Jin H, Wang H, Yuan J, Bao T, Jiang X, Zhang W, Zhao H, Yao L (2008) Expression of NDRG2 in clear cell renal cell carcinoma. Biol Pharm Bull 31:1316–1320. doi:JST.JSTAGE/bpb/31.1316
Murray JT, Campbell DG, Morrice N, Auld GC, Shpiro N, Marquez R, Peggie M, Bain J, Bloomberg GB, Grahammer F, Lang F, Wulff P, Kuhl D, Cohen P (2004) Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem J 384:477–488. doi:10.1042/BJ20041057
Oh SS, Kim D, Kim DH, Chang HH, Sohn KC, Kim KH, Jung SH, Lee BK, Kim JH, Kim KD (2012) NDRG2 correlated with favorable recurrence-free survival inhibits metastasis of mouse breast cancer cells via attenuation of active TGF-beta production. Carcinogenesis 33:1882–1888. doi:10.1093/carcin/bgs211
Philpott KL, McCarthy MJ, Klippel A, Rubin LL (1997) Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J Cell Biol 139:809–815. doi:10.1083/jcb.139.3.809
Shen L, Liu X, Hou W, Yang G, Wu Y, Zhang R, Li X, Che H, Lu Z, Zhang Y, Yao L (2010) NDRG2 is highly expressed in pancreatic beta cells and involved in protection against lipotoxicity. Cell Mol Life Sci 67:1371–1381. doi:10.1007/s00018-010-0258-1
Si R, Tao L, Zhang HF, Yu QJ, Zhang R, Lv AL, Zhou N, Cao F, Guo WY, Ren J, Wang HC, Gao F (2011) Survivin: a novel player in insulin cardioprotection against myocardial ischemia/reperfusion injury. J Mol Cell Cardiol 50:16–24. doi:10.1016/j.yjmcc.2010.08.017
Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G (2009) Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res 104:15–18. doi:10.1161/CIRCRESAHA.108.186429
Skyschally A, van Caster P, Iliodromitis EK, Schulz R, Kremastinos DT, Heusch G (2009) Ischemic postconditioning: experimental models and protocol algorithms. Basic Res Cardiol 104:469–483. doi:10.1007/s00395-009-0040-4
Sun Z, Shen L, Sun X, Tong G, Sun D, Han T, Yang G, Zhang J, Cao F, Yao L, Wang H (2011) Variation of NDRG2 and c-Myc expression in rat heart during the acute stage of ischemia/reperfusion injury. Histochem Cell Biol 135:27–35. doi:10.1007/s00418-010-0776-9
Takahashi K, Saitoh A, Yamada M, Iwai T, Inagaki M, Yamada M (2012) Dexamethasone indirectly induces Ndrg2 expression in rat astrocytes. J Neurosci Res 90:160–166. doi:10.1002/jnr.22727
Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, Ma XL (2007) Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation 115:1408–1416. doi:10.1161/CIRCULATIONAHA.106.666941
Tong G, Sun Z, Wei X, Gu C, Kaye AD, Wang Y, Li J, Zhang Q, Guo H, Yu S, Yi D, Pei J (2011) U50,488H postconditioning reduces apoptosis after myocardial ischemia and reperfusion. Life Sci 88:31–38. doi:10.1016/j.lfs.2010.10.018
Wang L, Liu N, Yao L, Li F, Zhang J, Deng Y, Liu J, Ji S, Yang A, Han H, Zhang Y, Han W, Liu X (2008) NDRG2 is a new HIF-1 target gene necessary for hypoxia-induced apoptosis in A549 cells. Cell Physiol Biochem 21:239–250. doi:10.1159/000113765
Wang L, Liu N, Yao L, Li F, Zhang J, Deng Y, Liu J, Ji S, Yang A, Han H, Zhang Y, Zhang J, Han W, Liu X (2008) NDRG2 is a new HIF-1 target gene necessary for hypoxia-induced apoptosis in A549 cells. Cell Physiol Biochem 21:239–250. doi:10.1159/000113765
Zhou RH, Kokame K, Tsukamoto Y, Yutani C, Kato H, Miyata T (2001) Characterization of the human NDRG gene family: a newly identified member, NDRG4, is specifically expressed in brain and heart. Genomics 73:86–97. doi:10.1006/geno.2000.6496
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81230043, 31171112, 81001161 and 81001123); National Basic Research Program of China (2012CB518101); Team Development Grant by China Department of Education (2010CXTD01) and China’s Ministry of Science and Technology 863 Program (2012AA02A603).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Z. Sun, G. Tong and N. Ma contributed equally to this work and should be considered as co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
395_2013_341_MOESM1_ESM.tif
Supplemental Fig. 1 Fluorescence microscopy showing the transduction efficiency in primary cardiomyocytes inoculated with Lenti-GFP for 72 h. Scale bar = 100 μm (Tiff 6.14 mb)
395_2013_341_MOESM2_ESM.tif
Supplemental Fig. 2 Local injection of lentivirus within myocardium and NDRG2 expression detection in different area after different treatment. (a) 2 × 109 TU/ml virus in 100 μl solution were divided into three equal volume and injected into 3 sites. In brief, 2 sites were on each side of the LAD, 2 mm lower than the future ligation site and 5 mm lateral to the LAD. The other site was the apex of heart. This pattern of injection was chosen to ensure universal distribution of virus in ischemic area-to-be. (b) Immunohistochemistry staining of NDRG2 protein in different areas of cardiac tissue. Scale bar = 25 um (c) Western blot analysis of NDRG2 expression in ischemic area and non-ischemic area post local lentivirus injection (Tiff 8.04 mb)
395_2013_341_MOESM3_ESM.tif
Supplemental Fig. 3 Representative image of immunofluorescence staining of CD11b (leukocyte marker) for myocardial inflammation detection after injection. Scale bar = 25 um (Tiff 4.22 mb)
395_2013_341_MOESM4_ESM.tif
Supplemental Fig. 4 Myocardial apoptosis, infarct size and cardiac function evaluation in rats subjected to I/R after NDRG2 knockdown. (a) Western blotting detection of NDRG2 expression in rat heart tissue after transduced with NDRG2 shRNA lentivirus or scramble shRNA lentivirus for 4 days. (b) Representative images of TUNEL-positive cardiomyocytes within ischemic area after different treatment. Scale bar = 50 um (c) The apoptotic index is expressed as the percentage of TUNEL-positive myocytes (up panel) over total nuclei determined by DAPI staining (low panel). (d) Representative illustrations of infarct size as stained by Evans Blue and TTC and statistical analysis of INF/AAR and AAR/LV values of two groups. (e) Representative M-mode images by echocardiography from rats 72 h after I/R injury and statistical analysis of LVEF and LVFS values of two groups (Tiff 2.79 mb)
395_2013_341_MOESM5_ESM.tif
Supplemental Fig. 5 Effect of NDRG2 knockdown on the cardiomyocytes survival within the border area after insulin treatment. (a) Immunohistochemistry evaluation of NDRG2 expression within border area. Scale bar = 50 um (b–c) Representative images of TUNEL-positive cardiomyocytes within border area after injected with different lentivirus and statistical analysis of the apoptotic rate. Scale bar = 50 um (Tiff 5.54 mb)
395_2013_341_MOESM6_ESM.tif
Supplemental Fig. 6 Comparison of apoptotic indexes and proteins levels between sham operation group and I/R group in vivo as well as between control group and SI/R group in vitro. (a) Western blotting detection of active-caspase3 expression in rat heart tissue. (b) Immunohistochemical staining of active-caspase3 protein in rat heart tissue. Scale bar = 50 um (c) Representative images of TUNEL-positive cardiomyocytes in rat heart tissue and statistical analysis of the apoptotic rate. Scale bar = 50 um **P < 0.01 versus sham operation group (d) Western blotting detection of both total and phosphorylated Akt and NDRG2 expression in rat heart tissue. (e) Western blotting detection of both total and phosphorylated Akt and NDRG2 expression in cultured cardiomyocytes. (f) Western blotting detection of active-caspase3 expression in cultured cardiomyocytes. (c) Representative images of TUNEL-positive cultured cardiomyocytes and statistical analysis of the apoptotic rate. Scale bar = 50 um **P < 0.01 versus control group (Tiff 2.94 mb)
395_2013_341_MOESM7_ESM.tif
Supplemental Table 1 Basic hemodynamics parameters of rats before surgery (baseline), 30 min post ischemia and 1 h post reperfusion. Values are presented as mean ± SEM (Tiff 792 kb)
Rights and permissions
About this article
Cite this article
Sun, Z., Tong, G., Ma, N. et al. NDRG2: a newly identified mediator of insulin cardioprotection against myocardial ischemia–reperfusion injury. Basic Res Cardiol 108, 341 (2013). https://doi.org/10.1007/s00395-013-0341-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-013-0341-5