Abstract
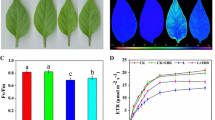
The effects of γ-aminobutyric acid (GABA) treatment on parameters of photosynthesis and antioxidant defense system were measured in pepper (Capsicum annuum L.) leaves under low-light (LL) stress. Seedlings exposed to LL stress showed increased chlorophyll content as well as decreased net photosynthetic rate (P n), stomatal conductance (g s), maximum quantum yield of PSII (F v/F m), actual PSII photochemical efficiency (ΦPSII), electron transport rates and photochemical quenching coefficient (q p). However, almost all the photosynthetic parameters above were enhanced markedly in seedlings treated with GABA under LL stress. Moreover, LL stress increased malondialdehyde (MDA) content, superoxide anion radical (O2 ·−) and hydrogen peroxide (H2O2) production. GABA-treated, LL-stressed seedlings exhibited lower MDA, O2 ·− and H2O2 production, and showed an activated antioxidant defense system, including increased activities of superoxide dismutase, catalase, ascorbate peroxidase, glutathione peroxidase, monodehydroascorbate reductase, dehydroascorbate reductase, glutathione reductase, ascorbate and glutathione. Moreover, seedlings subjected to LL stress showed increased endogenous GABA levels, and the level was further improved by application of exogenous GABA. These results suggest that GABA mitigates the LL-induced stress via regulating the antioxidant defense system and maintaining a high level of photochemical efficiency in pepper seedlings.





Similar content being viewed by others
References
Akihiro T, Koike S, Tani R, Tominaga T, Watanabe S, Iijima Y, Aoki K, Shibata D, Ashihara H, Matsukura C, Akama K, Fujimura T, Ezura H (2008) Biochemical mechanism on GABA accumulation during fruit development in tomato. Plant Cell Physiol 49:1378–1389
Anjum NA, Umar S, Iqbal M, Khan NA (2011) Cadmium causes oxidative stress in mung bean by affecting the antioxidant enzyme system and ascorbate–glutathione cycle metabolism. Russ J Plant Physiol 58:92–99
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396
Barbosa JM, Singh NK, Cherry JH, Locy RD (2010) Nitrate uptake and utilization is modulated by exogenous γ-aminobutyric acid in Arabidopsis thaliana seedlings. Plant Physiol Biochem 48:443–450
Bouche N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9:110–115
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Cao YY, Yang MT, Chen SY, Zhou ZQ, Li X, Wang XJ, Bai JG (2015) Exogenous sucrose influences antioxidant enzyme activities and reduces lipid peroxidation in water-stressed cucumber leaves. Biol Plant 59:147–153
Cockshull KE, Graves CJ, Cave RJ (2015) The influence of shading on yield of glasshouse tomatoes. J Hortic Sci 67:11–24
Crookston RK, Treharne KJ, Ludford P, Ozbun JL (1975) Response of beans to shading. Crop Sci 15:412–416
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Djanaguiraman M, Devi DD, Shanker AK, Sheeba JA, Bangarusamy U (2005) Selenium-an antioxidative protectant in soybean during senescence. Plant Soil 272:77–86
Doulis AG, Debian N, Kingstonsmith AH, Foyer CH (1997) Differential localization of antioxidants in maize leaves. Plant Physiol 114:1031–1037
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Flexas J, Ribas-Carbo M, Diaz-Espejo A, Galmes J, Medrano H (2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ 31:602–621
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Fu W, Li P, Wu Y (2012) Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci Hortic 135:45–51
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gill SS, Khan NA, Tuteja N (2011) Differential cadmium stress tolerance in five Indian mustard (Brassica juncea L.) cultivars. Plant Signal. Behavior 6:293–300
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212
Hasanuzzaman M, Hossain MA, Fujita M (2012) Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol Trace Elem Res 149:248–261
Hikosaka K (1996) Effects of leaf age, nitrogen nutrition and photon flux density on the organization of the photosynthetic apparatus in leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Planta 198:144–150
Hoque MA, Banu MNA, Okuma E, Amako K, Nakamura Y, Shimoishi Y, Murata Y (2007) Exogenous proline and glycinebetaine increase NaCl-induced ascorbate–glutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco Bright Yellow-2 suspension-cultured cells. J Plant Physiol 164:1457–1468
Hu XH, Xu ZR, Xu WN, Li JM, Zhao N, Zhou Y (2015) Application of γ-aminobutyric acid demonstrates a protective role of polyamine and GABA metabolism in muskmelon seedlings under Ca(NO3)2 stress. Plant Physiol Biochem 92:1–10
Janssen LHJ, Wams HE, Hasselt PRV (1992) Temperature dependence of chlorophyll fluorescence induction and photosynthesis in tomato as affected by temperature and light conditions during growth. J Plant Physiol 139:549–554
Kappel F, Flore JA (1983) Effect of shade on photosynthesis, specific leaf weight, leaf chlorophyll content, and morphology of young peach trees. J Am Soc Hortic Sci 108:541–544
Khoo HK, Ismail A, Mohd-Esa N, Idris S (2008) Carotenoid content of underutilized tropical fruits. Plant Foods Hum Nutr 63:170–175
Kinnersley AM, Turano FJ (2000) Gamma aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci 19:479–509
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem J 210:899–903
Lillo C (1994) Light regulation of nitrate reductase in green leaves of higher plants. Physiol Plant 90:616–620
Liu JL, Wang WX, Wang LY, Sun Y (2015) Exogenous melatonin improves seedling health index and drought tolerance in tomato. Plant Growth Regul 77:317–326
Luo YL, Su ZL, Bi TJ, Cui XL, Lan QY (2014) Salicylic acid improves chilling tolerance by affecting antioxidant enzymes and osmoregulators in sacha inchi (Plukenetia volubilis). Braz J Bot 37:357–363
Malekzadeh P, Khara J, Heydari R (2014) Alleviating effects of exogenous Gamma-aminobutiric acid on tomato seedling under chilling stress. Physiol Mol Biol Plants 20:133–137
Mazzucotelli E, Tartari A, Cattivelli L, Forlani G (2006) Metabolism of gamma-aminobutyric acid during cold acclimation and freezing and its relationship to frost tolerance in barley and wheat. J Exp Bot 57:3755–3766
Miyashita Y, Good AG (2008) Contribution of the GABA shunt to hypoxia-induced alanine accumulation in roots of Arabidopsis thaliana. Plant Cell Physiol 49:92–102
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nayyar H, Kaur R, Kaur S, Singh R (2014) γ-aminobutyric acid (GABA) imparts partial protection from heat stress injury to rice seedlings by improving leaf turgor and upregulating osmoprotectants and antioxidants. J Plant Growth Regul 33:408–419
Oda Y (1997) Effeets of light intensity, CO2 concentration and leaf temperature on gas exehange of strawberry Plants-Feasibility studies on CO2 enrichment in Japanese conditions. Acta Hortiecultrae 439:563–574
Oxborough K (2004) Imaging of chlorophyll a fluorescence: theoretical and practical aspects of an emerging technique for the monitoring of photosynthetic performance. J Exp Bot 55:1195–1205
Patterson BD, Macrae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem 139:487–492
Pereira GJG, Molina SMG, Lea PJ, Azevedo RA (2002) Activity of antioxidant enzymes in response to cadmium in Crotalaria juncea. Plant Soil 239:123–132
Perez M, Invers O, Ruiz JM, Frederiksen MS, Holmer M (2007) Physiological responses of the seagrass Posidonia oceanica to elevated organic matter content in sediments: an experimental assessment. J Exp Mar Biol Ecol 344:149–160
Perveen S, Anis M, Aref IM (2013) Lipid peroxidation, H2O2 content, and antioxidants during acclimatization of Abrus precatorius to ex vitro conditions. Biol Plant 57:417–424
Praba ML, Vanangamudi M, Thandapani V (2004) Effect of low light on yield and physiological attributes of rice. Int Rice Res Notes 29:71–73
Ramiro DA, Guerreiro-Filho O, Mazzafera P (2006) Phenol contents, oxidase activities, and the resistance of coffee to the leaf miner Leucoptera coffeella. J Chem Ecol 32:1977–1988
Renault H, Roussel V, Amrani AE, Arzel M, Renault D, Bouchereau A, Deleu C (2010) The Arabidopsis pop2-1 mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant Biol 10:157–160
Scott-Taggart CP, Cauwenberghe ORV, Mclean MD, Shelp BJ (1999) Regulation of γ-aminobutyric acid synthesis in situ by glutamate availability. Physiol Plant 106:363–369
Shalata A, Mittova V, Volokita M, Guy M, Tal M (2001) Response of the cultivated tomato and its wild salt-tolerant relative Lycopersiconpennellii to salt-dependent oxidative stress: the root antioxidative system. Physiol Plant 112:487–494
Shang HT, Cao SF, Yang ZF, Cai YT, Zheng YH (2011) Effect of exogenous γ-aminobutyric acid treatment on proline accumulation and chilling injury in peach fruit after long-term cold storage. J Agric Food Chem 59:1264–1268
Shelp BJ, Bown AW, Mclean MD (1999) Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 4:446–452
Smirnoff N (1993) The role of active oxygen in the response to water deficit and desiccation. New Phytol 125:27–58
Song YJ, Diao QN, Qi HY (2014) Putrescine enhances chilling tolerance of tomato (Lycopersicon esculentum Mill.) through modulating antioxidant systems. Acta Physiol Plant 36:3013–3027
Tanaka K, Otsubo T, Kondo N (1982) Participation of hydrogen peroxide in the inactivation of Calvin-Cycle SH enzymes in SO2-fumigated spinach leaves. Plant Cell Physiol 23:1009–1018
Turgeon R (1989) The sink-source transition in leaves. Ann Rev Plant Physiol Plant Mol Biol 40:119–138
Kooten OV, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Vijayakumari K, Puthur JT (2015) γ-Aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. plants subjected to PEG-induced stress. Plant Growth Regul 78:1–11
Wan YY, Zhang Y, Zhang L, Zhou ZQ, Li X, Shi QH, Wang XJ, Bai JG (2015) Caffeic acid protects cucumber against chilling stress by regulating antioxidant enzyme activity and proline and soluble sugar contents. Acta. Physiol Plant 37:1–10
Wang CY, Fan LQ, Gao HB, Wu XL, Li JR, Lv GY, Gong BB (2014) Polyamine biosynthesis and degradation are modulated by exogenous gamma-aminobutyric acid in root-zone hypoxia-stressed melon roots. Plant Physiol Biochem 82:17–26
White AJ, Critchley C (1999) Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth Res 59:63–72
Yang AP, Cao SF, Yang ZF, Cai YT, Zheng YH (2011) γ-Aminobutyric acid treatment reduces chilling injury and activates the defence response of peach fruit. Food Chem 129:1619–1622
Yin YQ, Yang RQ, Gu ZX (2014) Calcium regulating growth and GABA metabolism pathways in germinating soybean (Glycine max L.) under NaCl stress. Eur Food Res Technol 239:149–156
Youn YS, Park JK, Jang HD, Rhee YW (2012) Sequential hydration with an-aerobic and heat treatment increases GABA (γ-aminobutyric acid) content in wheat. Food Chem 129:1631–1635
Yu C, Zeng LZ, Sheng K, Chen FX, Zhou T, Zheng XD, Yu T (2014) γ-Aminobutyric acid induces resistance against Penicillium expansum by priming of defence responses in pear fruit. Food Chem 159:29–37
Zhang J, Zhang Y, Du Y, Chen S, Tang H (2011) Dynamic metabonomic responses of tobacco (Nicotiana tabacum) plants to salt stress. J Proteome Res 10:1904–1914
Zhao GJ, Bown AW (1997) The rapid determination of gamma-aminobutyric acid. Phytochemistry 44:1007–1009
Zhao DL, Oosterhuis DM (1998) Influence of shade on mineral nutrient status of field-grown cotton. J Plant Nutr 21:1681–1695
Zhu XG, Ort DR, Whitmarsh J, Long SP (2004) The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. J Exp Bot 55:1167–1175
Acknowledgements
We thank the language work of editor with native English background in Elixigen Corporation. This work was financially supported by the President-Aohongbozhe Fund of Jinzhou Medical University (No. XXJJ20140124), the Natural Science Fund of Liaoning Province (No. 2015020797), and Liaoning Engineering Research Center of Meat Processing and Quality Safety Control.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Fan, Y., Ma, Y. et al. Effects of Exogenous γ-Aminobutyric Acid (GABA) on Photosynthesis and Antioxidant System in Pepper (Capsicum annuum L.) Seedlings Under Low Light Stress. J Plant Growth Regul 36, 436–449 (2017). https://doi.org/10.1007/s00344-016-9652-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9652-8