Abstract
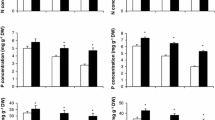
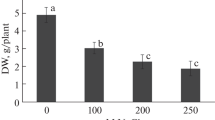
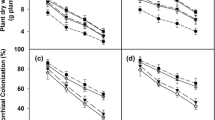
No information is available concerning effective microorganisms’ (EM) influence on the ROS-scavenging system under saline conditions. Thus, as a first approach, the present study evaluates the effect of EM on antioxidant machinery, and also visualizes the interrelationship between EM and salinity stress response components. Phaseolus vulgaris cv. Nebraska plants were grown under non-saline or saline conditions (2.5 and 5.0 dS m−1) with and without EM application. Plants exposed to soil salinity exhibited a significant decline in growth, productivity and membrane stability index. However, the follow-up treatment with EM detoxified the stress generated by salinity and significantly improved the above parameters. The concentrations of proline and glycinebetaine, the activities of antioxidative enzymes (superoxide dismutase, peroxidase, catalase and glutathione reductase), and the contents of antioxidant molecules (glutathione, ascorbate, phenols and anthocyanins) were increased under saline conditions; these increases were more significant in salt-stressed plants treated with EM. Soil salinization induced oxidative damage through increased lipid peroxidation, electrolyte leakage and hydrogen peroxide levels. EM application altered plant physiology and significantly reduced the oxidative damage. Both the prevention of oxidative stress and the elimination of ROS can be one of the most effective mechanisms used by EM-treated plants to gain tolerance against salinity stress. Indeed, with this biological strategy plants find protection from the deleterious effects of oxidants and cope with salty soils.






Similar content being viewed by others
References
Abdel-Aal ESM, Huel PA (1999) Rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheat. Cereal Chem 76:350–354
Alscher PG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plant. J Exp Bot 53:1331–1341
Anderson ME (1985) Determination of glutathione and glutathione disulfides in biological samples. Methods Enzymol 113:548–570
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Physiol Plant Mol Biol 55:373–399
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Bates LS, Aldren RP, Teare LD (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bayuelo-Jiménes JS, Craig R, Lynch JP (2002) Salinity tolerance of phaseolus species during germination and early seedling growth. Crop Sci 42:1584–1594
Beauchamp CO, Fridovich I (1971) Superoxide dismutase: improved assays and assay applicable to acrylamide gels. Ann Biochem 44:276–287
Chalker-Scott L (2002) Do anthocyanins function as osmoregulators in leaf tissues? Adv Bot Res 37:103–106
Chance B, Maehly AC (1955) Assay of catalase and peroxidases. Methods Enzymol 2:764–775
Cottenie A, Verloo M, Kiekens L, Velghe G, Camerlynck R (1982) Chemical analysis of plants and soils. Laboratory of Analytical and Agrochemistry, State University, Ghent, pp 14–24
D’Souza MR, Devaraj VR (2010) Biochemical responses of Hyacinth bean (Lablab purpureus) to salinity stress. Acta Physiol Plant 32:341–353
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Higa T (2000) What is EM technology? EM World J 1:1–6
Higa T (2004) Effective microorganisms—a new dimension for nature farming. In: Parr JF, Hornick SB, Simpson ME (eds) Proceedings of the 2nd international nature farming conference. U.S. Department of Agriculture, Washington, DC, pp 20–22
Hodges MD, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hu C, Qi Y (2013) Long-term effective microorganisms application promote growth and increase yields and nutrition of wheat in China. Eur J Agron 46:63–67
Ismail SM (2013) Influence of effective microorganisms and green manure on soil properties and productivity of pearl millet and alfalfa grown on sandy loam in Saudi Arabia. Afr J Microbiol Res 7:375–382
Jana S, Choudhari MA (1981) Glycolate metabolism of three submerged aquatic angiosperms during ageing. Aquat Bot 12:345–354
Kovácik J, Backor M (2007) Phenylalanine ammonia-lyase and phenolic compounds in chamomile tolerance to cadmium and copper excess. Water Air Soil Poll 185:185–193
Läuchli A (1984) Salt exclusion: an adaptation of legumes for crops and pastures under saline conditions. In: Staples RC, Toenniessen GH (eds) Salinity tolerance in plants: strategies for crop improvement. Wiley, New York, pp 155–159
Law MY, Charles SA, Halliwelll B (1983) Glutathione and ascorbic acid in spinach (Spinach oleracea) chloroplasts. The effect of hydrogen peroxide and paraquat. Biochem J 210:899–903
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398
Mallik S, Nayak M, Sahu AK, Shaw BP (2011) Response of antioxidant enzymes to high NaCl concentration in different salt tolerant plants. Biol Plant 55:191–195
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Noctor G, Foyer C (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Parida AK, Das AB (2005) Salt tolerance and salinity effect of plants: a review. Ecotoxicol Environ Saf 60:324–349
Porcel R, Aroca R, Ruı´z-Lozano JM (2012) Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron Sustain Dev 32:181–200
Sairam RK (1994) Effects of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture stress conditions of two wheat varieties. Plant Growth Regul 14:173–181
Sairam RK, Tyagi A (2004) Physiological and molecular biology of salinity stress tolerance in plants. Curr Sci 86:407–420
Schenck zu Schweinsberg-Mickan M, Müller T, (2009) Impact of effective microorganisms and other biofertilizers on soil microbial characteristics, organic-matter decomposition, and plant growth. J Plant Nutr Soil Sci 172:704–712
Sheng M, Tang M, Chen H, Yang BW, Zhang FF, Huang YH (2008) Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 18:287–296
Smith LK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,5-thiobis (2-nitrobenzoic acid). Anal Biochem 175:408–413
Snedecor GW, Cochran WG (1980) Statistical methods, 7th edn. Iowa State University Press, Ames Iowa
Szabados L, Savoure´ A (2009) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Talaat NB, Shawky BT (2013) 24-Epibrassinolide alleviates salt-induced inhibition of productivity by increasing nutrients and compatible solutes accumulation and enhancing antioxidant system in wheat (Triticum aestivum L.). Acta Physiol Plant 35:729–740
Talaat NB, Shawky BT (2014a) Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ Exp Bot 98:20–31
Talaat NB, Shawky BT (2014b) Modulation of the ROS-scavenging system in salt-stressed wheat plants inoculated with arbuscular mycorrhizal fungi. J Plant Nutr Soil Sci 177:199–207
Thompson JE, Froese CD, Madey E, Smith MD, Hong Y (1988) Lipid metabolism during plant senescence. Prog Lipid Res 37:119–141
Tsuda T, Shiga K, Ohshima K, Kawakishi S, Osawa T (1996) Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L. Biochem Pharmacol 52:1033–1039
Yang X, Liang Z, Wen X, Lu C (2008) Genetic engineering of the biosynthesis of glycinebetaine leads to increased tolerance of photosynthesis to salt stress in transgenic tobacco plants. Plant Mol Biol 66:73–86
Yildiz-Aktas L, Dagnon S, Gurel A, Gesheva E, Edreva A (2009) Drought tolerance in cotton: involvement of non-enzymatic ROS scavenging compounds. J Agron Crop Sci 195:247–253
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talaat, N.B. Effective Microorganisms Improve Growth Performance and Modulate the ROS-Scavenging System in Common Bean (Phaseolus vulgaris L.) Plants Exposed to Salinity Stress. J Plant Growth Regul 34, 35–46 (2015). https://doi.org/10.1007/s00344-014-9440-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-014-9440-2