Abstract
Polyamines (PAs) belong to plant growth regulators and in complex with classical phytohormones take part in regulation of seed dormancy and germination. Although the impact of reactive oxygen (ROS) and nitrogen (RNS) species on seed germination is well described, the cross talk of PAs with ROS/RNS has never been analyzed. Due to the close connection of PAs and ethylene biosynthetic pathways to arginine (Arg)-dependent NO biosynthesis we investigated production of nitric oxide (NO), peroxynitrite (ONOO−) and the level of O •−2 or H2O2 in apple embryos, germination of which was PA regulated. PAs: putrescine (Put) and spermidine (Spd) in contrast to spermine (Spm) stimulated germination of apple embryos. Among amino acids, stimulation of germination was observed in Arg and ornithine (Orn) only. Dormancy removal of embryos by PAs was associated with increased accumulation of H2O2 and O •−2 in embryonic axes. At the same stage of completion of sensu stricto germination the stimulatory effect of PAs (Put and Spd) and amino acids, mainly Arg and Orn, was accompanied by enhanced NO and ONOO− production in embryonic axis. The beneficial effect of PAs (Put and Spd) and their precursors on germination of apple embryos was removed by NO scavenging, suggesting a crucial role of NO in termination of embryo germination and radicle growth. Moreover, activity of polyamine oxidase in embryo axes was greatly enhanced by embryo fumigation with NO. Our data demonstrate the interplay of RNS/ROS with PAs and point to NO action as an integrator of endogenous signals activating germination.
Similar content being viewed by others
Introduction
Polyamines (PAs) are low molecular weight aliphatic cations. They are widespread in diverse organisms including higher plants and act as modulators of many developmental and physiological processes particularly reactions to stresses (Baron and Stasolla 2008; Kusano and others 2008; Takahashi and Kakehi 2010). In plants, putrescine (Put) is a major diamine and a direct substrate for triamine—spermidine (Spd) and tetraamine—spermine (Spm) formation. Put is synthesized from arginine (Arg) in reaction catalyzed by Arg decarboxylase or from ornithine (Orn) by Orn decarboxylase. Synthesis of Spd or Spm (Fig. 1) requires decarboxylated S-adenosylmethionine (dcSAM). SAM is a molecule central to ethylene biosynthesis, because it is synthesized from methionine (Met), and acts as substrate for formation of 1-aminocyclopropane-1-carboxylic acid (ACC)—a direct precursor of ethylene (Wang and others 2002; Bűrnsterbinder and Sauter 2012). Therefore, a competition between PAs and ethylene biosynthesis is suggested. Additionally, PAs oxidases (PAO) (EC 1.5.3.11), catalyzing the reaction of oxidative deamination of Spm and Spd, also act as a source of H2O2 (Bais and Ravishankar 2002; Cona and others 2003). Moreover, Arg operates as a nitric oxide (NO) precursor (Fig. 1). In plant cells synthesis of NO related to Arg, catalyzed by NO synthase-like (NOS-like) enzyme, is still under discussion, as so far no NOS-like protein has been isolated in higher plants. Foresi and others (2010) isolated and characterized NOS in green alga Ostreococcus tauri. To date, NOS-like activity (in contrast to protein) associated with NO and l-citrulline formation from l-Arg has been detected in various plant materials (Misra and others 2010; Talwar and others 2012; Mur and others 2013). The evidence that PAs induce production of NO was presented several years ago in seedlings of Arabidopsis thaliana (Tun and others 2006). This effect was abolished by 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO)—a commonly used NO scavenger. The network of biosynthetic pathways of ethylene, PAs, and NO (Fig. 1) suggests a close interaction between these plant growth regulators (Yamasaki and Cohen 2006), and their putative interaction in regulation of many physiological processes, for example, senescence or seed germination.
Biosynthesis and catabolism of PAs in plant cells, linked to the ethylene biosynthetic pathway and Arg-dependent NO biosynthesis. According to Baron and Stasolla (2008), Cona and others (2008) with modifications. Abbreviation of metabolites: dcSAM decarboxylated SAM, SAM S-adenozylmethionine. Abbreviation of enzymes: ACO ACC oxidase, ACS ACC synthase, ADC arginine decarboxylase, AIH agmatine iminohydrolase, CPA aminohydrolase N-carbamoylputrescine, NOS-like NO synthase-like enzyme, ODC ornithine decarboxylase, PAO polyamine oxidase, SPDS Spd synthase, SPMS Spm synthase
Seed germination is considered as initiation of the first developmental phase in the life cycle of a higher plant. This process can be defined as events that commence with the uptake of water by the quiescent (non-dormant) dry seed and terminate with the elongation of the embryonic axis. Such a definition corresponds with the terms “germination sensu stricto” or “early germination” (Bewley 1997). Therefore, germination is a physiological stage in which growth is manifested by the emergence of the embryonic root (the radicle) through the endosperm and seed coat. Seed dormancy prevents the germination of intact viable seeds even during favorable conditions in an otherwise unfavorable season. This is a complex feature as it is influenced by both endogenous and environmental factors. Seeds can rapidly loose dormancy during prolonged imbibition at the specific circumstances, for example, high or low temperatures. Regulation of seed dormancy and germination depends on the activity of several endogenous factors including classical phytohormones such as gibberellins (GAs) (Kucera and others 2005), abscisic acid (ABA) (Kermode 2005), or ethylene (Matilla and Matilla-Vazquez 2008; Bogatek and Gniazdowska 2012); plant growth regulators, for example, PAs (Matilla 1996); and signaling molecules such as reactive oxygen (ROS) or reactive nitrogen (RNS) species (Bailly and others 2008; Širová and others 2011). Complicated mechanisms that control seed dormancy at a molecular level have been summarized recently by Graeber and others (2012). NO or its donors are known to stimulate germination of seeds of various plant species (Širová and others 2011; Gniazdowska and others 2012). NO has been proposed as a key player in the ABA and ethylene cross talk in seeds (Arc and others 2013). In apple (Malus domestica) embryos, the enhancement of embryo germination by a range of NO donors was accompanied by elevated ethylene production, decreased ABA sensitivity (Gniazdowska and others 2007, 2010a), and transient ROS accumulation (Gniazdowska and others 2010b). The role of ROS and RNS in regulation of seed germination is well described by the model of “oxidative window” or “nitrosative door,” respectively (Bailly and others 2008; Krasuska and Gniazdowska 2012). Under natural conditions dormancy of apple seeds is removed by 90-day-long cold (5 °C) stratification (Lewak 2011). Such treatment leads to alterations in GAs, ABA, ethylene, and PA content (Lewak 2011 and references therein), which are accompanied by fluctuation in H2O2 concentration in the tissue and NO production (Dębska and others 2013).
Although much emphasis has been placed on PAs, the role of ROS or RNS in regulation of dormancy and germination of seeds, and the complexity of their interaction have never been discussed. Therefore, the aim of our work was to investigate the impact of three PAs (Put, Spd, and Spm) and their precursors (amino acids: Arg, Orn, and Met) on dormancy alleviation of apple embryos in relation to the regulatory role of ROS (H2O2 and O •−2 ) and RNS (NO and ONOO−) during sensu stricto germination, by measurement of ROS and RNS level/production. Additionally, as PA catabolism depending on PAO activity results in production of H2O2, we investigated NO-induced modification of PAO activity, to examine PAs cross talk with ROS and RNS in the axes of germinating apple embryos. To our knowledge the presented data are absolutely only one of their kind, as no more than Tun and others (2006) demonstrated a direct impact of PAs on NO generation, whereas Yamasaki and Cohen (2006) and recently Wimalasekera and others (2011) presented a hypothesis linking PAs and NO metabolism.
Materials and Methods
Plant Material
The experiments were performed using apple (M. domestica Borkh, cv. Antonówka) seeds harvested in 2010–2011. Fruits of apple were obtained from the Kordel fruit producer at Tarczyn (Poland). Dormant seeds were stored in dark glass containers at 5 °C. Seed coat and endosperm were removed from seeds imbibed for 24 h in distilled water at room temperature (20 °C). All determinations were conducted on the isolated embryos.
Germination Tests
The impact of Pas (Put, Spd, and Spm) and PA precursors (Arg, Orn, and Met) on germination of apple embryos was checked using a water solution of the tested chemicals at 0.1–0.5 mM concentrations.
Control (dormant, non-treated) embryos and embryos treated with PAs or PA precursors were germinated for 14 days in 9 cm Petri dishes (15 embryos per dish) in a Sanyo growing chamber (Versalite Environmental Test Chamber MLR-35OH) at 25/20 °C with a 12/12 h (light/dark) photoperiod, under a light intensity 150 μmol PAR m−2 s−1.
Experiments with inhibitors of PA synthesis: L-canavanine (Can)—analog of Arg and DFMO (difluoromethylornithine hydrochloride hydrate, inhibitor of Orn decarboxylase) at 0.1, 0.5, and 1 mM concentrations were also performed. Dormant embryos were subjected to DFMO or Can water solution for 14 days and cultured in a growing chamber as described above.
Additional treatments of apple embryos were done with vapors of acidified nitrite (NO) as described by Gniazdowska and others (2010b). Acidified nitrite was prepared using 20 mM sodium nitrite (NaNO2) and 0.2 M HCl. After 3 h of NO fumigation embryos were rinsed in distilled water and placed in 9 cm Petri glass dishes containing filter paper wetted with 5 ml distilled water. Embryos pretreated with NO were used only to determine RNS (NO and ONOO−) production and germination tests described below.
To test the simultaneous effect of NO and Arg or Spm on regulation of embryonal dormancy, apple embryos pretreated with NO (as described above) were subjected to imbibition in Arg (0.1 mM) or Spm (0.3 mM) water solution for 14 days.
Moreover, to provide evidence of a role for NO in PA-dependent dormancy removal, the simultaneous effect of PAs and the NO scavenger—cPTIO was determined by germination trials performed with 0.3 mM cPTIO and PAs at the concentration that exhibited the best effect on germination: Put (0.1 mM) and Spd (0.2 mM). In this experiment Spm (0.3 mM) at the lowest concentration leading to inhibition of germination to the greatest extend was used. Similar research was done with cPTIO (0.3 mM) and amino acids at a concentration that exhibited the best effect on germination: Arg (0.1 mM), Met (0.1 mM), or Orn (0.2 mM). In this experiment embryos germinated in a water solution of the appropriate PA or amino acid, respectively, were considered as a control. All germination tests were conducted for 14 days in a growth chamber, as described above.
Embryos were considered to have germinated when radicles were 2–3 mm long and exhibited typical gravitropic bending. This physiological stage of seeds was defined as termination of germination sensu stricto.
Germination tests were done in 5 independent experiments, in each 45 embryos were used (3 Petri dishes containing 15 embryos each). Embryos after 2 days of culture and at the morphological stage of completion of sensu stricto germination, when characteristic gravitropic bending of radicles was shown, were used for biochemical determinations.
Measurement of Hydrogen Peroxide (H2O2) Concentration
The concentration of H2O2 in the axes of isolated embryos was determined according to the method of Velikova and others (2000) with some modifications as described previously by Gniazdowska and others (2010b). Embryos germinated in PAs or amino acids at the concentration that exhibited the best effect on germination [Put (0.1 mM), Spd (0.2 mM) and Arg (0.1 mM), Orn (0.2 mM) and Met (0.1 mM)] or significantly inhibited germination—[Spm (0.3 mM)], and control (dormant) embryos after 2 days of culture and at the physiological stage of termination of germination sensu stricto were used for the experiment. Plant material (10 isolated embryo axes, ~15 mg) was homogenized on ice with 0.1 % (w/v) cold TCA and then centrifuged at 15,000×g for 15 min at 4 °C. The H2O2 concentration was measured spectrophotometrically at 390 nm using a Hitachi U-2900 spectrophotometer, in the assay mixture containing: 0.25 ml supernatant, 1 ml freshly prepared 1 M KI in 10 mM potassium phosphate buffer (pH 7.0), and 0.5 ml 10 mM potassium phosphate buffer (pH 7.0). Measurements of H2O2 concentration were done in three independent experiments, each in three biological replicates and expressed as μmol mg−1 FW.
Visualization of In Situ Localization of Superoxide Anion (O •−2 )
The embryos treated with PAs, at the concentration exhibiting the most spectacular impact on germination, [Put (0.1 mM), Spd (0.2 mM), Spm (0.3 mM)] or their precursors [Arg (0.1 mM), Orn (0.2 mM), Met (0.1 mM)], were incubated in 3 mM nitroblue tetrazolium (NBT) (Sigma-Aldrich) in 10 mM Tris–HCl buffer (pH 7.4) at room temperature for 15 min (Gniazdowska and others 2010b). The superoxide anion was visualized as a deposit of dark blue insoluble formazan compounds (Beyer and Fridovich 1987). Determinations were done using the whole intact embryos 2 days after starting the experiment.
Detection of NO and ONOO− in Embryo Axes
Production of NO or ONOO− in embryo axes was detected with some modifications as described previously by Gniazdowska and others (2010c) or by Gaupels and others (2011), respectively. The experiments were performed using axes of embryos isolated from embryos germinated in PAs or PA precursors after 2 days of treatment and at the physiological stage of termination of germination sensu stricto, when growth of embryo axes and their gravitropic bending were visible. NO or ONOO− production was determined in axes of embryos treated by tested substances at the concentration that exhibited the best effect on germination: Put (0.1 mM), Spd (0.2 mM), Arg (0.1 mM), Orn (0.2 mM), and Met (0.1 mM); or inhibited germination Spm (0.3 mM). Also, additional experiments were performed on axes isolated from embryos pretreated with acidified nitrite (NO) (Gniazdowska and others 2010b). For NO determination three isolated embryo axes (~0.005 g) were washed three times in 10 mM buffer HEPES–KOH (pH 7.4) and then incubated 1 h in darkness at 20 °C with 50 μL 20 μM fluorescent marker 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM DA, Invitrogen) in 10 mM buffer HEPES–KOH (pH 7.4). After the incubation, axes were washed 3 times in 10 mM buffer HEPES–KOH (pH 7.4) and transferred to the measurement cuvette containing 2,000 μL 10 mM HEPES–KOH (pH 7.4). For ONOO− determination three isolated embryo axes (~0.005 g) were washed three times in 100 mM K-phosphate buffer (pH 7.4) and then incubated 1 h in darkness at 20 °C with 500 μL APF (10 μM) (aminophenyl fluorescein, Invitrogen) in 100 mM K-phosphate buffer (pH 7.4). After the incubation, axes were washed 3 times in 100 mM K-phosphate buffer (pH 7.4) and transferred to the measurement cuvette containing 2,000 μL 100 mM K-phosphate buffer (pH 7.4). Fluorescence was recorded for 2,500 s (excitation 495 nm, emission 515 nm) using a Hitachi F-2500 spectrofluorometer and calculated per 1 mg FW.
Scavenging of ONOO− was detected after addition of uric acid in 3.36 mM NaOH in a final concentration of 1 mM in the measurement cuvette (according to Gaupels and others 2011) proceeded by 1-h incubation of the tissue in 2 mM uric acid.
All measurements were carried out in 6–8 repetitions, and their exact reproducibility was confirmed. Fluorescence was expressed in arbitrary units in comparison to fluorescence of control (axis of dormant embryos) estimated as 1 unit.
Determination of PAO Activity in Embryo Axes
Activity of PAO was determined as described by Luhová and others (2003) with some modifications. Axes (20 mg of fresh weight) of control or NO pretreated embryos (after 2 days of culture or at the stage of termination of germination sensu stricto) were washed in distilled water and homogenized in 2 ml extraction mixture containing 0.05 M K-phosphate buffer pH 6.5, 1 mM EDTA, 5 mM DTT, 0.1 mM PMSF, and 2 % PVPP in an ice bath. The homogenate was centrifuged at 15,000 g for 10 min at 4 °C. The supernatant (enzymatic source) was immediately used for determination of PAO activity. PAO activity was measured in 1 ml of reaction mixture containing 0.05 M K-phosphate buffer pH 6.5, 0.5 mM guaiacol (Sigma G5502), and 1 U of horseradish peroxidase (POX) in the presence of PAs—10 mM Spm or 10 mM Spd. Activity was detected as absorbance increase at 436 nm and measured using a Hitachi U-2900 spectrophotometer.
Protein concentration was measured according to Bradford (1970) using bovine serum albumin as a standard.
Statistical Analysis
Data were analyzed using the StatGraphics 5.1. Means were computed for each experiment and significance of differences was assessed with Tukey’s studentized range test or Duncan’s test. Differences were considered significant at P < 0.05.
Results
Germination of Dormant Apple Embryos is Modified by PAs, PA Precursors, and Inhibitors of PA Synthesis
Germination of dormant apple embryos was inhibited and delayed (Table 1). After 2 weeks of culture only about 10 % of the embryos germinated. In general PAs (accept 0.3 and 0.5 mM Spm) stimulated germination of apple embryos, and this effect was dose dependent. Spd was the most active among the PAs. The most pronounced effect of Spd was observed for the 0.2 mM concentration, in which almost 50 % of embryos germinated after 14 days. Moreover Spd accelerated embryos germination. Acceleration of embryo germination in reaction to PAs was also detected in Put (0.1 and 0.2 mM). Stimulation of embryo germination by Put was less evident as compared to Spd, but it should be underlined that 2 weeks of treatment with 0.2 mM Put resulted in a twofold stimulation of germination whereas 0.1 mM in a threefold higher rate of germination in comparison to control (Table 1). Among amino acids, the higher speed of germination was detected in 0.1 mM Arg and Orn at all the tested concentrations. Both amino acids enhanced germination of apple embryos, but in the case of Arg a better effect was detected at lower concentrations—mainly 0.1 mM, whereas for Orn an intermediate (0.2 mM) concentration. Stimulation of embryos germination by Arg (0.1 mM) and Orn (0.2 mM) was comparable to that induced by 0.1 mM Put and only slightly lower than by 0.2 mM Spd (Table 1). Met at the lower concentration (0.1 mM) resulted in only slight increase of germination rate after 14 days, and the positive effect of this amino acid even declined as the concentration increased.
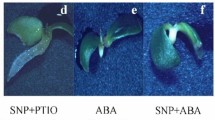
Despite restriction in growth of embryo axis, dormancy of apple embryo is also exhibited by typical anomalies in development and greening of cotyledons (Fig. 2). Establishment of the representative embryos or seedlings grown from dormant (non-treated embryos) and after dormant embryo treatment with PAs or amino acids is shown in Fig. 2.
Embryo germination and establishment of young seedlings in the presence of PAs (0.1 mM Put, 0.2 mM Spd, and 0.3 mM Spm) or amino acids (0.1 mM Arg, 0.2 mM Orn, and 0.1 mM Met). Dormant apple embryos imbibed in water (control) show typical asymmetric growth and greening of cotyledons and restriction in radicle elongation. Two-day imbibition in PAs or amino acids does not induce any visible alteration in germination of embryos, only light greening of lower cotyledon may be detected. Prolonged imbibition of embryos in 0.1 mM Put, 0.2 mM Spd and 0.1 mM Arg, 0.2 mM Orn resulted in radicle elongation as well as growth and greening of both cotyledons. Spm (0.3 mM) does not lead either to embryo germination, or to complete greening of upper cotyledon. Canavanine (0.5 mM) enhances embryo dormancy, inhibiting both radicle growth and greening of cotyledons. Images of the representative embryos are shown
DFMO, an inhibitor of PAs biosynthesis, did not inhibit apple embryo germination (Table 2). In the prolonged culture (longer than 10 days) it even stimulated embryo germination when applied at the higher concentration (0.5, 1 mM). The opposite effect was observed after Can (analog of Arg) treatment, which at 0.5 or 1 mM concentrations completely abolished germination of apple embryos (Table 2).
Dormancy of apple embryos was even deeper after treatment with cPTIO. Dormant embryos imbibed in cPTIO did not germinate, nor was any greening of cotyledons detected (data not shown). Simultaneous application of cPTIO and PAs at the concentration that exhibited the most pronounced effect on germination: Put (0.1 mM) and Spd (0.2 mM) resulted in removal of the beneficial effect of PAs on embryo germination (Table 3). Addition of cPTIO to Spm (0.3 mM) did not alter embryo dormancy. Germination of embryos dependent on Met and Orn was lowered about fivefold by the NO scavenger (Table 3 vs Table 1). The most negative effect of cPTIO was detected for embryos imbibed simultaneously in cPTIO and Arg. Embryos exposed to both Arg and the NO scavenger germinated in <3 % or did not germinate at all (Table 3).
Spm- or Arg-regulated germination of apple embryo was influenced by fumigation with NO. Pretreatment with NO followed by application of Arg resulted in only slight stimulation of germination as compared to Arg treatment (Table 3 vs Table 1). In contrast, fumigation with NO eliminated the effect of maintenance of dormancy by Spm, resulting in almost threefold higher germination, similar to that observed after Put or Spd application (Tables 1, 3).
PAs and Their Precursors Affect ROS Concentration in Embryonic Axis of Apple Embryos
H2O2 Concentration in Embryo Axes is Influenced by PAs and Amino Acids
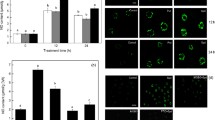
The H2O2 concentration in axes of dormant apple embryos was about 200 μmol g−1 FW after 2 days of germination in water (Fig. 3). It remained constant during the culture period. Among PAs the most visible effect on H2O2 level in embryonic axes was detected after Spd and Put treatment. The highest H2O2 concentration was observed in axes at the physiological stage of termination of germination sensu stricto, and it was almost twice that of the control dormant embryos and rose to approximately 375 μmol g−1 FW (Fig. 3). Spm did not influence H2O2 concentration in axes of embryos cultured for 2 days. Prolonged culture in Spm resulted in only a minor (in 25 %) increase in H2O2 concentration. All tested amino acids (Arg, Orn, and Met) stimulated H2O2 production in axes when embryo germination was terminated (Fig. 3). In such embryos, H2O2 concentration reached values of about 320–340 μmol g−1 FW. Moreover, at this physiological stage no significant differences in H2O2 concentration in axis of embryos treated with various amino acids were observed.
Concentration of H2O2 in axes of control dormant embryos germinating in water, in PAs (Put (0.1 mM), Spm (0.3 mM), and Spd (0. 2 mM)) or PA precursors (Arg (0.1 mM), Met (0.1 mM), and Orn (0.2 mM)) after 2 days of culture, and at the stage of termination of germination sensu stricto. Means of values are statistically different at P < 0.05 when they share no common letter(s). The comparisons were made using the Duncan test. Average of the three experiments ± SD
Superoxide Anion Concentration in Embryonic Axes depends on PA and Amino Acids Treatment
In situ accumulation of O •−2 in embryo sections through axes and cotyledons is presented at Fig. 4. At the whole-tissue level, accumulation of formazan occurred mostly in axes of Put-, Spd-, Arg-, and Orn-treated embryos. In contrast, NBT staining of embryos subjected to Met was only slight, and comparable to that detected in dormant, control embryos germinating in water (Fig. 4).
In situ visualization of superoxide anion (O •−2 ) in dormant (control) apple embryos, in embryos imbibed in PAs (Put (0.1 mM), Spd (0.2 mM), and Spm (0.3 mM)), amino acids (Arg (0.1 mM), Met (0.1 mM), and Orn (0.2 mM)) and in embryos shortly (3 h) pretreated with NO after 2 days of imbibition period
PAs and their Precursors Stimulate RNS Production in Embryonic Axes of Germinating Embryos
Put (0.1 mM) and Spd (0.2 mM) stimulated NO production at the beginning of the culture (after 2 days of imbibition) and at the physiological stage of radicle elongation (Fig. 5a). At that time NO production in axes of embryos treated with both PAs was fourfold higher as compared to the control. A comparable rate of NO production was detected in axes isolated from germinating embryos imbibed in Arg (0.2 mM). In contrast, Spm (0.3 mM), Orn (0.2 mM), and Met (0.1 mM) did not enhance NO production in axes of 2-day-old embryos but led to approximately doubled NO production at the completion of germination sensu stricto (mainly in the case of Spm and Orn) (Fig. 5a).
Production of NO (a) and ONOO− (b) in embryonic axes of apple embryos imbibed in water (control) or germinated in PAs: Put (0.1 mM), Spd (0.2 mM), and Spm (0.3 mM); and amino acids: Arg (0.1 mM), Orn (0.1 mM), and Met (0.1 mM) determined 2 days after starting the experiment and after completion of germination sensu stricto. Means of values are statistically different at P < 0.05 when they share no common letter(s). The comparisons were made using the Duncan test. Average of the 6–8 experiments ± SD
Production of ONOO− measured as APF fluorescence was significantly enhanced in axes of germinating embryos (at the physiological stage of termination of germination sensu stricto) (Fig. 5b). Among PAs that evidently stimulated germination, only Put (0.1 mM) led to an increased ONOO− level just at the beginning of the treatment (2 days of imbibition). After 2 days in axes of all the treated embryos, excluding Put and Arg (0.1 mM) only, production of ONOO− was comparable or even lower than that in axes of the control embryos (Fig. 5b).
Short-term pretreatment of dormant embryos with NO resulted in a great increase in NO production by the axes (data not shown). After 2 days of culture it was 10.5 ± 1.2 fluorescence units, more than 10 times higher as compared to the control. However, at the stage of termination of germination sensu stricto it dropped down and was 3.7 ± 0.5, equal to that observed in growing axes of embryos treated with Put, Spd, and Arg (Fig. 5a). In contrast, the ONOO− level in axes of NO pretreated embryos did not increase so evidently. It reached the value of 2.2 ± 0.3 and 2.1 ± 0.2 fluorescence units after 2 days of imbibition and at the stage of termination of germination, respectively. It was approximately doubled in comparison to the control and slightly lower to that observed for germinating axes of Put-, Spd-, and Arg-treated embryos (Fig. 5b).
To confirm that the observed increase in fluorescence was due to the presence of peroxynitrite, we introduced a peroxynitrite scavenger into the measurement systems. One mM uric acid almost totally (to 15–20 %) eliminated the increase in fluorescence induced by the tested chemicals (data not showed).
NO Stimulates Activity of PAO in Axes of Germinating Apple Embryos
Acivity of PAO in axes of control dormant embryos remained stable during the entire period of experiment (Table 4). In the contrast, activity of PAO in axes of germinating embryos pretreated with NO was tenfold higher than that in the control, non-treated embryos. A transient maximum of PAO activity in axes of germinating embryos was observed at the beginning of the culture, 2 days after NO fumigation (Table 4). In NO pretreated embryos it decreased slightly at the stage of completion of germination sensu stricto, but was still threefold higher as compared to the control (Table 4).
Discussion
PAs are considered as a group of important growth regulators taking part in regulation of seed dormancy and germination, in both undisturbed and stressed conditions (for example, salinity) (Benavides and others 1997). During seed development (embryogenesis) and germination sensu stricto characteristic fluctuations of PA concentrations in embryonic axes are observed, although the content of particular PAs and seed sensitivity to them may vary, depending on the plant species (Krasuska and others 2012). In the present study the impact of PAs and their precursors on the apple embryo germination was investigated in relation to the NO beneficial effect on dormancy alleviation. Embryos isolated from dormant apple seeds did not germinate or germinated very slowly, due to blockage of many physiological processes in the embryonic axes, and unbalanced plant phytohormone levels (Lewak 2011 and references therein). Dormancy of apple seeds may be overcome by cold stratification (Lewak 2011) or, in the case of isolated embryos, by application of various NO donors (Gniazdowska and others 2010b). We demonstrated that the most abundant PAs (Put and Spd) stimulated germination of dormant apple embryos similarly as it was described by Sińska and Lewandowska (1991). However, in our experiment a lower concentration of PAs and an extended period of culture (to 14 days) were needed. Germination of apple embryos in the presence of the selected amino acids such as Arg, Met, and Orn was also dose dependent. The highest embryo germination was observed when Arg (0.1 mM) was applied, whereas the lowest was when Met was present in the imbibition medium. Moreover, Met concentrations above 0.1 mM inhibited apple embryo germination. It is well known that certain amino acids (including Met) at millimolar concentrations inhibit seed germination due to misregulation of metabolism (Vurro and others 2006). In our study Arg supplementation probably stimulated the apple embryo germination by both enhancement of NO production and PAs biosynthesis.
DFMO, an inhibitor of Orn decarboxylase, one of the enzymes of the PA biosynthetic pathway, did not inhibit germination of dormant embryos, but in contrast led to a threefold stimulation of germination. Therefore, in other experiments DFMO was not applied as a PA biosynthesis inhibitor. It should also be noticed that stimulation of germination by DFMO was similar to that observed after embryo imbibition in Orn or Put solution. In contrast, Can a structural analog of Arg, at concentrations higher than 0.1 mM, led to the total inhibition of embryo germination. Embryos treated with Can did not germinate during the whole period of experiments. Moreover, they were white with no greening of cotyledons. The presence of Can, in the germination medium, at concentrations higher than 1 mM completely blocked metabolism and as a consequence led to the death of apple embryos. Therefore, it may be assumed that Can at concentrations lower than 1 mM enhanced dormancy of apple embryos. The negative effect of Can was not visible in embryos pretreated with NO (data not shown). Embryos subjected to NO and then imbibed in Can germinated well, similar to that observed after NO treatment only (Gniazdowska and others 2010b, c), suggesting a close relationship between Arg and NO biosynthesis and the dominant role of NO in embryo dormancy alleviation. Spm is known to be accumulated in seeds during embryogenesis and responsible for enhanced dormancy status; in addition, Spm-resistant mutants display precocious germination (Mirza and Rehman 1998). Germination of apple embryos was generally not affected or even slightly inhibited by Spm at all the tested concentrations. Embryos subjected to Spm stay in dormancy status as long as they are exposed to this PA. This information corresponds well with the previous data presented by Sińska and Lewandowska (1991). But, the most important, pretreatment with NO followed by imbibition in Spm (0.3 mM) resulted in more than threefold stimulation of embryo germination, which highlights the beneficial role of NO in regulation of seed germination. Moreover, it can be assumed that NO or other RNS take part in PA catabolism, especially in the degradation of Spm. Non-direct NO involvement in Spm degradation can be observed as increased PAO activity in the axis of embryos fumigated by this gas. These findings correspond well to the recently proposed model of “nitrosative door,” describing a key role of RNS in seed dormancy alleviation and germination (Krasuska and Gniazdowska 2012).
It was demonstrated previously that NO scavenging by cPTIO affected the apple embryo germination and led to complete inhibition of this process (Gniazdowska and others 2010c). Simultaneous exposure of apple embryos to PAs (except Spm) or amino acids (Arg, Orn, and Met) and cPTIO resulted in lowering germination in 80–90 % as compared to germination induced by the related PA or PA precursor, implying a direct involvement of NO in PA-mediated dormancy removal. Pretreatment of embryos with NO proceeding culture in Arg solution stimulated germination of embryos only in 20 % in comparison to embryos imbibed in Arg only. So we may suspect that: in the presence of exogenous Arg, NOS-like dependent generation of NO may operate and is sufficient for dormancy alleviation and embryo transition into the non-dormant stage. NO is produced by germinating seeds, at the highest level at the third phase of this process, when radicle protrusion is detected. Moreover, treatment of seeds with the various NO donors release dormancy and accelerate germination, even in stressful environmental conditions (Kopyra and Gwóźdź 2003; Giba and others 2007). Alleviation of embryonic dormancy of apple during cold stratification is associated with the enhanced production of NO, mainly at the terminal phase of the treatment. During the early germination of embryos isolated from fully stratified (non-dormant) seeds, the burst of NO was detected (Dębska and others 2013). PAs, which induced germination of apple embryos, also enhanced RNS production in embryonic axes. The highest NO and ONOO− production in apple axes was detected in embryos imbibed in Put and Spd: PAs exhibiting the greatest effect on germination. However, NO production in the axis of embryos imbibed in PAs or amino acids for 2 days was not as high as that observed after NO short pretreatment. In general, enhanced RNS production (NO and ONOO−) seems to be a characteristic for axes exhibiting gravitropic bending and correlates with the elongation growth of radicles. This observation corresponds well with data showing a positive relationship between gravistimulation and NO production in roots (Hu and others 2005) and the supposed role of NO as an emerging regulator of cell elongation during the primary root growth (Fernandez-Marcos and others 2012).
The direct link between PAs and NO was demonstrated both in physiological development or stress conditions, which is not surprising, when comparing the cellular function of PAs and NO (Wimalasekera and others 2011). Similar to our data it was shown that embryonic cells of Araucaria angustifolia produced more NO after Put treatment, as compared to Spm or Spd (Silveira and others 2006). However, in contrast to our findings, the highest NO production in cucumber (Cucumis sativus) leaves exposed to drought stress was induced after pretreatment with Spm and Spd (Arasimowicz-Jelonek and others 2009). Nevertheless, in germinating apple embryos NO may be considered as a potential mediator of PA action whereas concentrations of other RNS (ONOO−) may be influenced, for example, by activity of PA oxidation by PAO leading to production of ROS.
The understanding of ROS involvement in seed dormancy release comes from many publications; for example for wheat, it was shown that the antioxidant defense pathway is associated with the maintenance of dormancy (Bykova and others 2011). Also some other factors have been used to break dormancy artificially, only in experiments conducted in the laboratory; for example, hydrogen cyanide (HCN) as a stimulator of germination of non-cyanogenic sunflower (Helianthus annus) seeds acted via ROS (Oracz and others 2009). Likewise, the apple embryo germination (apple seeds accumulate cyanogenic compounds) induced both by HCN or NO donors was dependent on the transient accumulation of ROS (Gniazdowska and others 2010b), accompanied by modification in the activity of ROS modulating enzymes (Krasuska and Gniazdowska 2012). We detected an increased ROS (H2O2 and O •−2 ) production in the axes of embryos germinating in Put and Spd, both at the beginning of this process (after 2 days of imbibitions), as well as during termination of sensu stricto germination. Higher ROS content is postulated as a part of the cell wall-loosening mechanism. Reduction of H2O2 with O •−2 leads to the formation of the hydroxyl radical (.OH) (Haber–Weiss reaction) which participates in cell wall-loosening (Chen and Schopfer 1999). Modulation of ROS metabolism in the apoplast is achieved by the activity of peroxidases or PAOs. PAOs in contrast to peroxidases (containing Fe) could be less sensitive to NO. PAOs are also involved in the cell elongation mechanism (Cona and others 2003). An increased concentration of H2O2 was also characteristic for axes of embryos whose germination was induced by Arg, Met, and Orn, mainly at the stage of radicle elongation and gravitropic bending. Such an observation corresponds well with the hypothesis of the “oxidative window” (Bailly and others 2008). It was proposed quite recently that ROS-dependent germination of many seeds is regulated by the activity of NADPH oxidase (Oracz and others 2009), which seems to be considered as an autopropagator of the ROS signal (Marino and others 2012). ROS-producing NADPH oxidase AtrbohB promoted seed afterripening in A. thaliana, and Atrboh mutants were not able to germinate (Müller and others 2009). The correlation between PAs and the activity of NADPH oxidase in seeds was not investigated so far but in the microsomal fraction of tobacco (Nicotiana tabacum) leaves PAs significantly altered NADPH oxidase-mediated superoxide generation (Papadakis and Roubelakis-Angelakis 2005). The activity of NADPH oxidase was inhibited by PAs (Spm > Spd > Put) applied at high concentrations (1–5 mM). Spm inhibited the activity of this enzyme by more than 70 %. Therefore, we may suspect that the observed negative effect of Spm on germination of apple embryos may be due to its action on NADPH activity and, in consequence, ROS production. In contrast, Put at the lower (0.5 mM) concentration stimulated activity of this enzyme (Papadakis and Roubelakis-Angelakis 2005), which may also partly explain the stimulation of the apple embryo germination. Interaction between ROS and phytohormone levels seems to be the most important event during germination sensu stricto and early growth of seedlings (Barba-Espin and others 2011). H2O2 could act, directly or indirectly, in the embryo, impairing ABA transport or/and inducing a decrease in ABA (Barba-Espin and others 2010). Similar variations in ABA concentration, transcription of the signaling pathway and catabolism were also detected in the apple embryos briefly fumigated with NO (non published data) in which ROS production raised transiently at the initial stage of germination.
It is not questionable that seed dormancy may be removed by PAs, although there is some variation in the fluctuation of PA contents during germination, and the type of PA, mostly active as enhancers of germination, varied depending on the plant species (Krasuska and others 2012 and references herein). The presented data suggest that the beneficial effect of some PAs (Put and Spd) on dormancy alleviation and germination of apple embryos is due to enhancement in ROS and RNS (mainly NO) production. Although the tight cross talk of PA-RNS-ROS is evident the origin of the evolved NO as well as the impact of PA on ROS metabolism needs further investigations.
References
Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Kubiś J (2009) Interaction between polyamine and nitric oxide signaling in adaptive responses to drought in cucumber. J Plant Growth Regul 28:177–186
Arc E, Sechet J, Corbineau F, Rajjou L, Marion-Poll A (2013) ABA cross talk with ethylene and nitric oxide in seed dormancy and germination. Front Plant Sci. doi:10.3389/fpls.2013.00063
Bailly C, El-Maarouf-Bouteau H, Corbineau F (2008) From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biol 331:806–814
Bais HP, Ravishankar GA (2002) Role of polyamines in the ontogeny of plants and their biotechnological applications. Plant Cell Tiss Org Cult 69:1–34
Barba-Espin G, Diaz-Vivancos P, Clemente-Moreno MJ, Albecete A, Faize L, Faize M, PérezAlfocea F, Hernández JA (2010) Interaction between hydrogen peroxide and plant hormones during germination and the early growth of pea seedlings. Plant Cell Environ 33:981–994
Barba-Espin G, Diaz-Vivancos P, Job D, Belghazi M, Job C, Hernández JA (2011) Understanding the role of H2O2 during pea seed germination: a combined proteomic and hormone profiling approach. Plant Cell Environ 34:1907–1919
Baron K, Stasolla C (2008) The role of polyamines during in vivo and in vitro development. In Vitro Cell Dev Biol Plant 44:384–395
Benavides MP, Aizencang G, Tomaro ML (1997) Polyamines in Helianthus annuus L. during germination under salt stress. J Plant Growth Regul 16:205–211
Bewley JD (1997) Seed germination and dormancy. Plant Cell 9:1055–1066
Beyer WF, Fridovich I (1987) Assaying for superoxide oxidase dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bogatek R, Gniazdowska A (2012) Ethylene in seed development, dormancy and germination. Annu Plant Rev 44:189–218
Bradford MM (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Bűrnsterbinder K, Sauter M (2012) Early events in the ethylene biosynthetic pathway—regulation of the pools of methionine and S-adenosylmethionine. Annu Plant Rev 44:19–52
Bykova NV, Hoehn B, Rampitsch C, Junjie H, Stebbing J, Knox R (2011) Thiol redox-sensitive seed proteome in dormant and non dormant genotypes of wheat. Phytochemistry 72:1162–1172
Chen S, Schopfer P (1999) Hydroxyl-radical production in physiological reactions, a novel function of peroxidase. Eur J Biochem 260:726–735
Cona A, Cenci F, Cervelli M, Federico R, Mariottini P, Moreno S, Angelini R (2003) Polyamine oxidase, a hydrogen peroxide producing enzyme, is up-regulated by light and down-regulated by auxin in the outer tissues of the maize mesocotyl. Plant Physiol 131:803–813
Cona A, Rea G, Angelini R, Federico R, Tavladoraki P (2008) Functions of amine oxidases in plant development and defense. Trends Plant Sci 11:80–88
Dębska K, Krasuska U, Budnicka K, Bogatek R, Gniazdowska A (2013) Dormancy removal of apple seeds by cold stratification is associated with fluctuation in H2O2, NO production and protein carbonylation level. J Plant Physiol 170:480–488
Fernandez-Marcos M, Sanz L, Lorenzo O (2012) Nitric oxide: an emerging regulator of cell elongation during primary root growth. Plant Signal Behav 2:1–7
Foresi N, Correa-Aragunde N, Parisi G, Caló G, Salerno G, Lamattina L (2010) Characterization of a nitric oxide synthase from the plant kingdom: NO generation from the green alga Ostreococcus tauri is light irradiance and growth phase dependent. Plant Cell 22:3816–3830
Gaupels F, Spiazzi-Vandelle E, Yang D, Delledonne M (2011) Detection of peroxynitrite accumulation in Arabidopsis thaliana during the hypersensitive defense response. Nitric Oxide 25:222–228
Giba Z, Grubišić D, Konjević R (2007) Seeking the role of NO in breaking seed dormancy. In: Lamattina L, Polacco JC (eds) Nitric oxide in plant growth, development and stress physiology plant cell monographs, vol 5. Springer, Berlin, pp 91–111
Gniazdowska A, Dobrzyńska U, Babańczyk T, Bogatek R (2007) Breaking of apple embryo dormancy by NO involves the stimulation of ethylene production. Planta 225:1051–1057
Gniazdowska A, Krasuska U, Bogatek R (2010a) Dormancy removal in apple embryos by nitric oxide or cyanide involves modifications in ethylene biosynthetic pathway. Planta 232:1397–1407
Gniazdowska A, Krasuska U, Czajkowska K, Bogatek R (2010b) Nitric oxide, hydrogen cyanide and ethylene are required in the control of germination and undisturbed development of young apple seedlings. Plant Growth Regul 61:75–85
Gniazdowska A, Krasuska U, Dębska K, Andryka P, Bogatek R (2010c) The beneficial effect of small toxic molecules on dormancy alleviation and germination of apple embryos is due to NO formation. Planta 232:999–1005
Gniazdowska A, Babańczyk T, Krasuska U (2012) Nitric oxide as germination controlling factor in seeds of various plant species. Phyton-Ann Rei Bot A 52:193–200
Graeber K, Nakabayashi K, Miatton E, Leubner-Metzger G, Soppe WJJ (2012) Molecular mechanisms of seed dormancy. Plant Cell Environ 35:1769–1786
Hu X, Neill SJ, Tang Z, Cai W (2005) Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol 137:663–670
Kermode AR (2005) Role of abscisic acid in seed dormancy. J Plant Growth Regul 24:319–344
Kopyra M, Gwóźdź EA (2003) Nitric oxide stimulates seed germination and counteracts the inhibitory effects of heavy metals and salinity on root growth of Lupinus luteus. Plant Physiol Biochem 41:1011–1017
Krasuska U, Gniazdowska A (2012) Nitric oxide and hydrogen cyanide as regulating factors of enzymatic antioxidant system in germinating apple embryos. Acta Physiol Plant 34:683–692
Krasuska U, Budnicka K, Bogatek R, Gniazdowska A (2012) Polyamines in regulation of seed dormancy and germination. Adv Cell Biol 39:395–414 (in Polish)
Kucera B, Cohn MA, Leubner-Matzger G (2005) Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15:281–307
Kusano T, Berberich T, Tateda C, Takahashi Y (2008) Polyamines: essential factors for growth and survival. Planta 228:367–381
Lewak S (2011) Metabolic control of embryonic dormancy in apple seed: seven decades of research. Acta Physiol Plant 33:1–24
Luhová L, Lebeda A, Hedererová D, Peč P (2003) Activities of amine oxidase, peroxidase and catalase in seedlings of Pisum sativum L. under different light conditions. Plant Soil Environ 49:151–157
Marino D, Dunand C, Puppo A, Pauly N (2012) A burst of plant NADPH oxidases. Trends Plant Sci 17:9–15
Matilla AJ (1996) Polyamines and seed germination. Seed Sci Res 6:81–93
Matilla AJ, Matilla-Vazquez MA (2008) Involvement of ethylene in seed physiology. Plant Sci 175:87–89
Mirza JI, Rehman A (1998) A spermine-resistant mutant of Arabidopsis thaliana displays precocious germination. Acta Physiol Plant 20:235–240
Misra AN, Misra M, Singh R (2010) Nitric oxide biochemistry, mode of action and signaling in plants. J Med Plants Res 4:2729–2739
Müller K, Carstens AC, Linkies A, Torres MA, Leubner-Metzger G (2009) The NADPH-Oxidase AtrbohB plays a role in Arabidopsis seed after-ripening. New Phytol 184:885–897
Mur LAJ, Mandon J, Persijn S, Cristescu SM, Moshkov IE, Novikova GV, Hall MA, Harren FJM, Hebelstrup KH, Gupta KJ (2013) Nitric oxide in plants: an assessment of the current state of knowledge. AoB PLANTS 5: pls052; doi:10.1093/aobpla/pls052
Oracz K, El-Maarouf-Bouteau H, Kranner I, Bogatek R, Courbineau F, Bailly C (2009) The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiol 150:494–505
Papadakis A, Roubelakis-Angelakis K (2005) Polyamines inhibit NADPH oxidase-mediated superoxide generation and putrescine prevents programmed cell death induced by polyamine oxidase-generated hydrogen peroxide. Planta 220:826–837
Silveira V, Santa-Catarina C, Tun NN, Schrer GFE, Handro W, Guerra MP, Floh EIS (2006) Polyamines effects on the endogenous polyamine contents, nitric oxide release, growth and differentiation of embryonic suspension cultures of Araucaria angustifolia (Bert.) O. Ktze. Plant Sci 171:91–98
Sińska I, Lewandowska U (1991) Polyamines and ethylene in the removal of embryonal dormancy in apple seeds. Physiol Plant 81:59–64
Širová J, Sedlářová M, Piterková J, Luhová L, Petřivalský M (2011) The role of nitric oxide in germination of plant seeds and pollen. Plant Sci 181:560–572
Takahashi T, Kakehi J-I (2010) Polyamines: ubiquitous polycations with unique roles in growth and stress responses. Ann Bot 105:1–6
Talwar PS, Gupta R, Maurya AK, Deswal R (2012) Brassica juncea nitric oxide synthase like activity is stimulated by PKC activators and calcium suggesting modulation by PKC-like kinase. Plant Physiol Biochem 60:157–164
Tun N, Santa-Catarina C, Begum T, Silveira V, Handro W, Floh E, Scherer GF (2006) Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol 47:346–354
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci 151:59–66
Vurro M, Boari A, Pilgeram AL, Sands DC (2006) Exogenous amino acids inhibit seed germination and tubercle formation by Orobanche ramosa (Broomrape): potential application for management of parasitic weeds. Biol Control 36:258–265
Wang KL-C, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell 14:S131–S151
Wimalasekera R, Tebartz F, Scherer GFE (2011) Polyamines, polyamines oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci 181:593–603
Yamasaki H, Cohen MF (2006) NO signal at the crossroads: polyamine-induced nitric oxide synthesis in plants? Trends Plant Sci 11:522–524
Acknowledgments
Work was financed by National Science Center Grant No. NN 303821840.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Krasuska, U., Ciacka, K., Bogatek, R. et al. Polyamines and Nitric Oxide Link in Regulation of Dormancy Removal and Germination of Apple (Malus domestica Borkh.) Embryos. J Plant Growth Regul 33, 590–601 (2014). https://doi.org/10.1007/s00344-013-9408-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-013-9408-7