Abstract
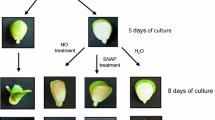
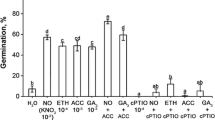
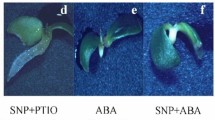
The connection between classical phytohormone-ethylene and two signaling molecules, nitric oxide (NO) and hydrogen cyanide (HCN), was investigated in dormancy removal and germination “sensu stricto” of apple (Malus domestica Borkh.) embryos. Deep dormancy of apple embryos was removed by short-term (3–6 h) pre-treatment with NO or HCN. NO- or HCN-mediated stimulation of germination was associated with enhanced emission of ethylene by the embryos, coupled with transient increase in ROS concentration in embryos. Ethylene vapors stimulated germination of dormant apple embryos and eliminated morphological anomalies characteristic for young seedlings developed from dormant embryos. Inhibitors of ethylene receptors completely impeded beneficial effect of NO and HCN on embryo germination. NO- and HCN-induced ethylene emission by apple embryo was only slightly reduced by inhibitor of 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase activity during first 4 days of germination. Short-term pre-treatment of the embryos with NO and HCN modified activity of both key enzymes of ethylene biosynthetic pathway: ACC synthase and ACC oxidase. Activity of ACC synthase declined during first 4 days of germination, while activity of ACC oxidase increased markedly at that time. Additional experiments point to non-enzymatic conversion of ACC to ethylene in the presence of ROS (H2O2). The results indicate that NO and HCN may alleviate dormancy of apple embryos “via” transient accumulation of ROS, leading to enhanced ethylene emission which is required to terminate germination “sensu stricto”. Therefore, ethylene seems to be a trigger factor in control of apple embryo dormancy removal and germination.






Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- ACO:
-
ACC oxidase
- ACS:
-
ACC synthase
- Aib:
-
α-Amino-isobutyric acid
- AVG:
-
Aminoethoxyvinylglycine
- cPTIO:
-
2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- HCN:
-
Hydrogen cyanide
- NBD:
-
Norbornadien
- NO:
-
Nitric oxide
- ROS:
-
Reactive oxygen species
- SAM:
-
S-Adenosyl-l-methionine
- SNP:
-
Sodium nitroprusside
- STS:
-
Silver thiosulfate
References
Abeles FB, Lonski J (1969) Stimulation of lettuce seed germination by ethylene. Plant Physiol 44:277–280
Bailly C, El-Maarouf-Bouteau H, Corbineau F (2008) From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biol 331:806–814
Beaudoin N, Serizet C, Gosti F, Giraudat J (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12:1103–1115
Bethke PC, Libourel IGL, Jones RL (2007) Nitric oxide in seed dormancy and germination. In: Bradford K, Nonogaki H (eds) Seed development, dormancy and germination. Blackwell, Oxford, pp 153–175
Bewley JD, Black M (1978) Physiology and biochemistry of seeds in relation to germination. Springer, New York
Bogatek R, Gniazdowska A (2006) Nitric oxide and HCN reduce deep dormancy of apple seeds. Acta Physiol Plant 28:281–287
Bogatek R, Dziewanowska K, Lewak ST (1991) Hydrogen cyanide and embryonal dormancy in apple seeds. Physiol Plant 83:417–421
Bogatek R, Gawrońska H, Oracz K (2003) Involvement of oxidative stress and ABA in CN-mediated elimination of embryonic dormancy in apple. In: Nicolas G, Bradford KJ, Come D, Pritchard HW (eds) The biology of seeds: recent research advances. CAB International Publishing, Wallingford, pp 211–216
Bogatek R, Sykała A, Krysiak C (2004) Cyanide-induced ethylene biosynthesis in dormant apple embryos. Acta Physiol Plant 26(Suppl):16
Borysjuk L, Macherel D, Benamar A, Wobus U, Rolletschek H (2007) Low oxygen sensing and balancing in plant seeds: a role for nitric oxide. New Phytol 176:813–823
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Calvo AP, Nicolas C, Lorenzo O, Nicolas G, Rodriguez D (2004a) Evidence for positive regulation by gibberellins and ethylene of ACC oxidase expression and activity during transition from dormancy to germination in Fagus sylvatica L. seeds. J Plant Growth Regul 23:44–53
Calvo AP, Nicolas C, Lorenzo O, Nicolas G, Rodriguez D (2004b) Evidence of a cross-talk regulation of a GA20-oxidase (FsGA20ox1) by gibberellins and ethylene during the breaking of dormancy in Fagus silvatica seeds. Physiol Plant 120:623–630
Chojnowski M, Corbineau F, Côme D (1997) Physiological and biochemical changes induced in sunflower seeds by osmopriming and subsequent drying, storage and aging. Seed Sci Res 7:323–332
Corbineau F, Bagniol S, Côme D (1990) Sunflower (Helianthus annuus L.) seed and its regulation by ethylene. Israel J Bot 39:313–325
Esashi Y, Leopold AC (1969) Dormancy regulation in subterranean clover seeds by ethylene. Plant Physiol 44:1470–1472
Esashi Y, Isuzugawa K, Matsuyama S, Ashino H, Hasegawa R (1991) Endogenous evolution of HCN during pre-germination periods in many seed species. Physiol Plant 83:27–33
Gallardo K, Job C, Groot SPC, Puype M, Demol H, Vandekerckhove J, Job D (2000) Importance of methionine biosynthesis for Arabidopsis seeds germination and seedling growth. Physiol Plant 116:238–247
Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12:1117–1126
Gniazdowska A, Dobrzyńska U, Babańczyk T, Bogatek R (2007) Breaking of apple embryo dormancy by nitric oxide involves stimulation of ethylene production. Planta 225:1051–1057
Gniazdowska A, Krasuska U, Czajkowska K, Bogatek R (2010) Nitric oxide, hydrogen cyanide and ethylene are required in the control of germination and undisturbed development of young apple seedlings. Plant Growth Regul 61:75–84
Gomez-Jimenez MC, Matilla AJ, Garrido D (1998) Isolation and characterization of a cDNA encoding an ACC oxidase from Cicer arietinum and its expression during embryogenesis and seed germination. Aust J Plant Physiol 25:765–773
Gomez-Jimenez MC, Garcia-Olivarez E, Matilla AJ (2001) 1-Amino-1-cyclopropane carboxylate oxidase from embryonic axis of germinating cheak-pea (Cicer arietinum L.) seeds: cellular immunolocalization and alteration in its expression by indole-3-acetic acid, abscisic acid and spermine. Seed Sci Res 11:243–253
Hall BP, Shakeel SN, Schaller GE (2007) Ethylene receptors: ethylene perceptional signal transduction. J Plant Growth Regul 26:118–130
Kacperska A, Kubacka-Zębalska M (1989a) Formation of stress ethylene depends both on ACC synthesis and on the activity of free radical-generating system. Physiol Plant 77:231–237
Kacperska A, Kubacka-Zębalska M (1989b) Stress ethylene metabolism as related to degree of tissue injury. In: Clijsters H, de Proft M, Marcelle M, van Poucke M (eds) Biochemical and physiological aspects of ethylene production in lower and higher plants. Kluwer Academic Publishers, Dordrecht, pp 211–218
Katoh H, Esashi Y (1975) Dormancy and impotency of coclebur seeds. I. CO2, C2H4, O2 and high temperature. Plant Cell Physiol 16:687–696
Kępczyński J, Karssen CM (1985) Requirement for the action of endogenous ethylene during germination of non-dormant seeds of Amaranthus caudatus. Physiol Plant 63:49–52
Kępczyński J, Kępczyńska E (1997) Ethylene in seed dormancy and germination. Physiol Plant 101:720–726
Kępczyński J, Rudnicki RM, Khan AA (1977) Ethylene requirement for germination of partly after-ripened apple embryo. Physiol Plant 40:292–295
Kępczyński J, Bihun M, Kępczyńska E (1996) Ethylene involvement in the dormancy and germination of Amaranthus seeds. In: Kanellis AK, Chang C, Kende H, Grierson D (eds) Proceedings of the international symposium on biology and biotechnology of the plant hormone ethylene. NATO ASI Series, Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 113–122
Ketring DL, Morgan PW (1971) Physiology of oil seeds II. Dormancy release in Virginia-type peanut seeds by plant growth regulators. Plant Physiol 47:488–492
Ketring DL, Morgan PW (1972) Physiology of oil seeds. IV. Role of endogenous ethylene and inhibitory regulators during natural and induced after ripening of dormant Virginia-type peanut seeds. Plant Physiol 50:382–387
Khan AA (1994) ACC-derived ethylene production, a sensitive test for seed vigor. J Am Soc Hort Sci 119:1083–1090
Kucera B, Cohn MA, Leubner-Metzger G (2005) Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15:281–307
Leubner-Metzger G, Petruzzelli L, Waldvogel R, Vögeli-Lange R, Meins F Jr (1998) Ethylene-responsive element binding protein (EREBP) expression and the transcriptional regulation of class I b-1,3-glucanase during tobacco seed germination. Plant Mol Biol 38:785–795
Linkies A, Muler K, Morris K, Tureckova V, Wenk M, Cadman CSC, Corbineau F, Strnad M, Lynn JR, Finch-Savage WE, Leubner-Metzger G (2009) Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell 21:3803–3822
Lizada CC, Yang SF (1979) A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem 100:140–145
Locke JM, Bryce JH, Morris PC (2000) Contrasting effects of ethylene perception and biosynthesis inhibitors on germination and seedling growth of barley (Hordeum vulgare L.). J Exp Bot 51:1843–1849
Lynch DV, Sridhara S, Thompson JE (1985) Lipoxygenase generated hydroperoxides account for the non-physiological features of ethylene formation from 1-aminocyclopropane-1-carboxylic acid by microsomal membranes of carnation. Planta 164:121–125
Machabee S, Saini HS (1991) Differences in the requirement for endogenous ethylene germination of dormant and non-dormant seeds of Chenopodium album L. J Plant Physiol 138:97–101
Mathooko FM, Kubo Y, Inaba A, Nakamura R (1993) Partial characterization of 1-aminocyclopropane-1-carboxylate oxidase from excised mesocarp tissue of winter squash fruit. Sci Rep Fac Agric Okayama Univ 82:49–59
Matilla AJ (2000) Ethylene in seed formation and germination. Seed Sci Res 10:111–126
Matilla AJ, Matilla-Vazquez MA (2008) Involvement of ethylene in seed physiology. Plant Sci 175:87–97
Nonogaki H, Chen F, Bradford KJ (2007) Mechanisms and genes involved in germination sensu stricto. In: Bradford KJ, Nonogaki H (eds) Seed development dormancy and germination. Blackwell, Oxford, pp 264–304
Oracz K, El-Maarouf Bouteau H, Farrant JM, Cooper K, Belghazi M, Job C, Job D, Corbineau F, Bailly C (2007) ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J 50:452–465
Oracz K, El-Maarouf Bouteau H, Bogatek R, Corbineau F, Bailly C (2008) Release of sunflower seed dormancy by cyanide: cross-talk with ethylene signaling pathway. J Exp Bot 59:2241–2251
Oracz K, El-Maarouf Bouteau H, Kranner I, Bogatek R, Corbineau F, Bailly C (2009) The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiol 150:494–505
Peck SC, Kende H (1995) Sequential induction of ethylene biosynthetic enzymes by indole-3-acetic acid in etiolated peas. Plant Mol Biol 28:293–301
Petruzzelli L, Coraggio I, Leubner-Metzger G (2000) Ethylene promotes ethylene biosynthesis during pea seed germination by positive feedback regulation of 1-aminocyclopropane-1-carboxylic acid oxidase. Planta 211:144–149
Pierik R, Sasidharan R, Voesenek LACJ (2007) Growth control by ethylene: adjusting phenotypes to the environment. J Plant Growth Regul 26:188–200
Puga-Hermida MI, Gallardo M, Rodrigez-Gacio MC, Matilla AJ (2006) Polyamine contents, ethylene synthesis and BrACO2 expression during turnip germination. Biol Plant 50:574–580
Sarath G, Bethke PC, Jones R, Baird LM, Hou G, Mitchell RB (2006) Nitric oxide accelerates seed germination in warm season grasses. Planta 223:1154–1164
Satoh S, Esashi Y (1984) Identification and content of 1-malonylaminocyclopropanecarboxylic acid in germinating cocklebur seeds. Plant Cell Physiol 25:583–587
Sergiev I, Alexieva V, Karanov E (1997) Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt Rend Acad Bulg Sci 51:121–124
Siegień I, Bogatek R (2006) Cyanide action in plants—from toxic to regulatory. Acta Physiol Plant 28:483–497
Simontacchi M, Jasid S, Puntarulo S (2004) Nitric oxide generation during early germination of sorghum seeds. Plant Sci 167:839–847
Sisler EC, Serek M (2003) Compounds interacting with the ethylene receptor in plants. Plant Biol 5:473–480
Yip WK, Dong JG, Yang SF (1991) Purification and characterization of 1-aminocyclopropane-1-carboxylate synthase from apple fruits. Plant Physiol 95:251–257
Zagórski S, Lewak ST (1985) Germination of lettuce seeds promoted by red light, gibberellin or cyanide is differently affected by far red illumination and temperature. Acta Physiol Plant 7:65–70
Acknowledgments
This work was supported by grant N N303 0905 34 founded by the Ministry of Science and Higher Education, Poland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gniazdowska, A., Krasuska, U. & Bogatek, R. Dormancy removal in apple embryos by nitric oxide or cyanide involves modifications in ethylene biosynthetic pathway. Planta 232, 1397–1407 (2010). https://doi.org/10.1007/s00425-010-1262-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1262-2