Abstract
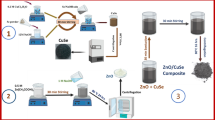
The amount of dopant concentration, alongwith the choice of dopant, is one of the most conducive factor for the favourable outcome for light driven activities of a material. The present paper reports on the synthesis of zinc oxide nanorods doped with different concentrations of copper (Cu–ZnO) by simple, low-cost mechanical assisted thermal decomposition process. The as synthesized samples were tested for visible light driven photo-electrochemical (PEC) and photocatalytic activities on various hazardous dyes using methylene blue (MB), methyl orange and mixed green dye (methyl thymol blue + methylene blue). The study helped us to reveal that highest degradation efficiency was achieved for Cu concentration of 5% in ZnO on MB (91.1% degradation in 40 min). Compared to pure ZnO, the photoactivity of 5% Cu–ZnO composites shows higher photodegradation of dyes. Moreover, the photocatalytic results were found consistent with PEC studies which showed maximum current generation of +9.4 mA for 5% Cu–ZnO (carried out under dark and illumination condition). The mechanism for this enhanced photoactivity has been proposed based on the relationship established between oxygen vacancies and defects generation in the material due to different doping concentrations that directly influence its photocatalytic efficiency.











Similar content being viewed by others
References
R. Xu, Beilstein J. Nanotechnol. 5(1), 1071–1072 (2014)
H. Chen, L. Wang, Beilstein J. Nanotechnol. 5(1), 696–710 (2014)
B. Du Ahn, S. H. Oh, C. H. Lee, G. H. Kim, H. J. Kim, S. Y. Lee, J. Cryst. Growth 309(2), 128–133 (2007)
E. Fortunato, L. Raniero, L. Silva, A. Goncalves, A. Pimentel, P. Barquinha, H. Aguas, L. Pereira, G. Goncalves, I. Ferreira, Sol. Energy Mater. Sol. Cells, 92(12), 1605–1610 (2008)
Y. Liu, Y. Hu, M. Zhou, H. Qian, X. Hu, Appl. Catal. B Environ. 125, 425–431 (2012)
K. Maeda, T. Takata, M. Hara, N. Saito, Y. Inoue, H. Kobayashi, K. Domen, J. Am. Chem. Soc. 127(23), 8286–8287 (2005)
K. Kakiuchi, E. Hosono, S. Fujihara, J. Photochem. Photobiol. A Chem. 179(1), 81–86 (2006)
N. H. Hong, J. Sakai, V. Brizé, J. Phys. Condens. Matter, 19(3), 36219 (2007)
C. He, T. Sasaki, H. Usui, Y. Shimizu, N. Koshizaki, J. Photochem. Photobiol. A Chem. 191(1), 66–73 (2007)
S. Kuriakose, V. Choudhary, B. Satpati, S. Mohapatra, Beilstein J. Nanotechnol 5(1), 639–650 (2014).
T. Bhuyan, K. Mishra, M. Khanuja, R. Prasad, A. Varma, Mater. Sci. Semicond. Process 32, 55–61 (2015)
X. Zhang, J. Qin, Y. Xue, P. Yu, B. Zhang, L. Wang, R. Liu, Sci. Rep. 4, 4596 (2014)
A. Sadollahkhani, I. Kazeminezhad, J. Lu, O. Nur, L. Hultman, M. Willander, RSC Adv 4, 36940–36950 (2014)
X. Zhang, J. Qin, L. Hao, R., Wang, X. Shen, R. Yu, S. Limpanart, M. Ma, R. Liu, J. Phys. Chem. C 119, 20544–20554 (2015)
D. Schelonka, M. Slušná, J. Tolasz, D. Popelková, P. Ecorchard, Mater. Today Proc. 3, 2679–2687 (2016)
C. Jagadish, S.J. Pearton, Zinc oxide bulk, thin films and nanostructures: processing, properties, and applications, 1st ed. (Elsevier Science, Kidlington, UK, 2006)
B. Yao, L.X. Guan, G.Z. Xing, Z.Z. Zhang, B.H. Li, Z.P. Wei, X.H. Wang, C.X. Cong, Y.P. Xie, Y.M. Lu, J. Lumin. 122, 191–194 (2007)
C.X. Xu, X.W. Sun, B.J. Chen, Appl. Phys. Lett. 84(9), 1540–1542 (2004)
X. Jiang, F.L. Wong, M.K. Fung, S.T. Lee, Appl. Phys. Lett. 83(9), 1875–1877 (2003)
M. Mahanti, D. Basak, J. Lumin. 145, 19–24 (2014)
W. Choi, A. Termin, M.R. Hoffmann, J. Phys. Chem. 98(51), 13669–13679 (1994)
G. Bystrzejewska-Piotrowska, J. Golimowski, P.L. Urban, Waste Manag. 29, 2587–2595 (2009)
M. Auffan, J. Rose, J.-Y. Bottero, G.V. Lowry, J.-P. Jolivet, M.R. Wiesner, Nat. Nanotechnol. 4, 634–641 (2009)
J. Gan, X. Lu, J. Wu, S. Xie, T. Zhai, M. Yu, Z. Zhang, Y. Mao, S.C.I. Wang, Y. Shen, Y. Tong, Sci. Rep 3, 1021 (2013)
X. Zhang, J. Qin, Y. Xue, P. Yu, B. Zhang, L. Wang, R. Liu, Sci. Rep 4, 4596 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S., Pendurthi, R., Khanuja, M. et al. Copper-doped modified ZnO nanorods to tailor its light assisted charge transfer reactions exploited for photo-electrochemical and photo-catalytic application in environmental remediation. Appl. Phys. A 123, 184 (2017). https://doi.org/10.1007/s00339-017-0806-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-017-0806-8