Abstract
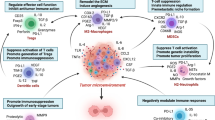
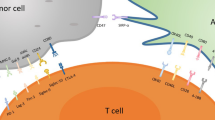
A bottleneck for immunotherapy of cancer is the immunosuppressive microenvironment in which the tumor cells are located. Regardless of the fact that large numbers of tumor-specific T cells can be generated in patients by active immunization or adoptive transfer, these T cells do not readily translate to tumor cell killing in vivo. The immune regulatory mechanism that prevents autoimmunity may be harnessed by tumor cells for the evasion of immune destruction. Regulatory T cells, myeloid-derived suppressor cells, inhibitory cytokines and immune checkpoint receptors are the major components of the immune system acting in concert with causing the subversion of anti-tumor immunity in the tumor microenvironment. This redundant immunosuppressive network may pose an impediment to efficacious immunotherapy, thus facilitating tumor progression. Cancer progression clearly documents the failure of immune control over relentless growth of tumor cells. Detailed knowledge of each of these factors responsible for creating an immunosuppressive shield to protect tumor cells from immune destruction is essential for the development of novel immune-based therapeutic interventions of cancer. Multipronged targeted depletion of these suppressor cells may restore production of granzyme B by CD8+ T cells and increase the number of IFN-γ-producing CD4+ T cells.

Similar content being viewed by others
Abbreviations
- AFP:
-
Alpha-fetoprotein
- Arg-1:
-
Arginase-1
- CCR4:
-
Chemokine receptor type-4
- COX2:
-
Cyclooxygenase-2
- CRC:
-
Colorectal cancer
- GARP:
-
Glycoprotein A repetition predominant
- GPC-3:
-
Glypican-3
- HCC:
-
Hepatocellular carcinoma
- MAGE-A:
-
Melanoma antigen-A
- mTOR:
-
Mammalian target of rapamycin
- NO:
-
Nitric oxide
- NY-ESO-1:
-
New York esophageal squamous cell cancer-1
- PDE-5:
-
Phosphodiesterase-5
- PKC:
-
Protein kinase C
- ROS:
-
Reactive oxygen species
- TAM:
-
Tumor-associated macrophages
- Th1:
-
T helper 1
- Tregs:
-
Regulatory T cells
References
Schrader J (2013) The role of MDSCs in hepatocellular carcinoma—in vivo veritas? J Hepatol 59:921–923
Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A et al (2013) The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int 2013:187204. doi:10.1155/2013/187204
Cabrera R, Szabo G (2013) Another armed CD4(+) T cell ready to battle hepatocellular carcinoma. Hepatology 58:1–3
Fu J, Zhang Z, Zhou L, Qi Z, Xing S, Lv J et al (2013) Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology 58:139–149
Pastille E, Bardini K, Fleissner D, Adamczyk A, Frede A, Wadwa M et al (2014) Transient ablation of regulatory T cells improves antitumor immunity in colitis-associated colon cancer. Cancer Res 74:4258–4269
Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF (2015) The yin and yang of evasion and immune activation in HCC. J Hepatol 62:1420–1429
Huang Y, Wang F, Wang Y, Zhu Z, Gao Y, Ma Z et al (2014) Intrahepatic interleukin-17+ T cells and FoxP3+ regulatory T cells cooperate to promote development and affect the prognosis of hepatocellular carcinoma. J Gastroenterol Hepatol 29:851–859
Zhang X, Kelaria S, Kerstetter J, Wang J (2015) The functional and prognostic implications of regulatory T cells in colorectal carcinoma. J Gastrointest Oncol 6:307–313
Kondo Y, Shimosegawa T (2015) Significant roles of regulatory T cells and myeloid derived suppressor cells in hepatitis B virus persistent infection and hepatitis B virus-related HCCs. Int J Mol Sci 16:3307–3322
Sharma S, Khosla R, David P, Rastogi A, Vyas A, Singh D et al (2015) CD4+CD25+CD127 (low) regulatory T cells play predominant anti-tumor suppressive role in hepatitis B virus-associated hepatocellular carcinoma. Front Immunol 6:49. doi:10.3389/fimmu.2015.00049
Bailur JK, Gueckel B, Derhovanessian E, Pawelec G (2015) Presence of circulating Her2-reactive CD8+ T-cells is associated with lower frequencies of myeloid-derived suppressor cells and regulatory T cells, and better survival in older breast cancer patients. Breast Cancer Res 17:34. doi:10.1186/s13058-015-0541-z
Zhang D, Chen Z, Wang DC, Wang X (2015) Regulatory T cells and potential inmmunotherapeutic targets in lung cancer. Cancer Metastasis Rev 34:277–290
Yan F, Du R, Wei F, Zhao H, Yu J, Wang C et al (2015) Expression of TNFR2 by regulatory T cells in peripheral blood is correlated with clinical pathology of lung cancer patients. Cancer Immunol Immunother 64:1475–1485
Kurose K, Ohue Y, Sato E, Yamauchi A, Eikawa S, Isobe M et al (2015) Increase in activated Treg in TIL in lung cancer and in vitro depletion of Treg by ADCC using an antihuman CCR4 mAb (KM2760). J Thorac Oncol 10:74–83
Talmadge JE, Gabrilovich DI (2013) History of myeloid-derived suppressor cells. Nat Rev Cancer 13:739–752
Stromnes IM, Greenberg PD, Hingorani SR (2014) Molecular pathways: myeloid complicity in cancer. Clin Cancer Res 20:5157–5170
Stromnes IM, Brockenbrough JS, Izeradjene K, Carlson MA, Cuevas C, Simmons RM et al (2014) Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 63:1769–1781
Mao Y, Poschke I, Wennerberg E, de Pico CY, Egyhazi BS, Schultz I et al (2013) Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res 73:3877–3887
Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H et al (2015) Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res 21:30–38
Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN et al (2012) IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology 1:1460–1468
Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM et al (2014) Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res 20:1601–1609
Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T et al (2013) Increase in CD14+ HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother 62:1421–1430
Napolitano M, D’Alterio C, Cardone E, Trotta AM, Pecori B, Rega D et al (2015) Peripheral myeloid-derived suppressor and T regulatory PD-1 positive cells predict response to neoadjuvant short-course radiotherapy in rectal cancer patients. Oncotarget 6:8261–8270
Idorn M, Kollgaard T, Kongsted P, Sengelov L, Thor SP (2014) Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol Immunother 63:1177–1187
Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y (2013) Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res 73:2435–2444
Medina-Echeverz J, Eggert T, Han M, Greten TF (2015) Hepatic myeloid-derived suppressor cells in cancer. Cancer Immunol Immunother 64:931–940
Diaz-Montero CM, Finke J, Montero AJ (2014) Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol 41:174–184
Zhu Y, Hawkins WG, DeNardo DG (2015) Regramming myeloid responses to improve cancer immunotherapy. Oncoimmunology 4:e974399. doi:10.4161/2162402X.2014.974399
Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH et al (2014) Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 20:5064–5074
Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH et al (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32:1020–1030
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R et al (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369:134–144
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Andersen MH (2014) The targeting of immunosuppressive mechanisms in hematological malignancies. Leukemia 28:1784–1792
Haile ST, Dalal SP, Clements V, Tamada K, Ostrand-Rosenberg S (2013) Soluble CD80 restores T cell activation and overcomes tumor cell programmed death ligand 1-mediated immune suppression. J Immunol 191:2829–2836
Ostrand-Rosenberg S, Horn LA, Haile ST (2014) The programmed death-1 immune-suppressive pathway: barrier to antitumor immunity. J Immunol 193:3835–3841
Woller N, Gurlevik E, Fleischmann-Mundt B, Schumacher A, Knocke S, Kloos AM et al (2015) Viral infection of tumors overcomes resistance to PD-1-immunotherapy by broadening neoantigenome-directed T-cell responses. Mol Ther 23:1630–1640
Schmidt N, Flecken T, Thimme R (2014) Tumor-associated antigen specific CD8 T cells in hepatocellular carcinoma—a promising target for immunotherapy. Oncoimmunology 3:e954919. doi:10.4161/21624011.2014.954919
Hato T, Goyal L, Greten TF, Duda DG, Zhu AX (2014) Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology 60:1776–1782
Lugade AA, Kalathil S, Miller A, Iyer R, Thanavala Y (2013) High immunosuppressive burden in advanced hepatocellular carcinoma patients: Can effector functions be restored? Oncoimmunology 2:e24679. doi:10.4161/onci.24679
Yano H, Thakur A, Tomaszewski EN, Choi M, Deol A, Lum LG (2014) Ipilimumab augments antitumor activity of bispecific antibody-armed T cells. J Transl Med 12:191. doi:10.1186/1479-5876-12-191
Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T et al (2014) Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2:632–642
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD et al (2015) Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 373:23–34
Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP et al (2004) Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res 10:4332–4341
Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R et al (2014) Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 59:1415–1426
Zhao HQ, Li WM, Lu ZQ, Yao YM (2014) Roles of Tregs in development of hepatocellular carcinoma: a meta-analysis. World J Gastroenterol 20:7971–7978
Santegoets SJ, Dijkgraaf EM, Battaglia A, Beckhove P, Britten CM, Gallimore A et al (2015) Monitoring regulatory T cells in clinical samples: consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry. Cancer Immunol Immunother 64:1271–1286
Lee IC, Huang YH, Chau GY, Huo TI, Su CW, Wu JC et al (2013) Serum interferon gamma level predicts recurrence in hepatocellular carcinoma patients after curative treatments. Int J Cancer 133:2895–2902
Acknowledgments
This research in the Thanavala Lab was supported in part through discretionary funds available to Dr. Thanavala. We gratefully acknowledge Dr. Paul Wallace and Earl Timm for their help in designing flow cytometry experiments. The patient samples were obtained through the Roswell Park Cancer Institute Data Bank and Biorepository which is a Cancer Center Support Grant (CCSG) Shared Resource supported by National Institute of Health P30 CA016056-27.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest.
Additional information
This paper is a Focussed Research Review based on a presentation given at the Fourth International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM 2015), held in Ljubljana, Slovenia, 27th–30th April 2015. It is part of a series of Focussed Research Reviews and meeting report in Cancer Immunology, Immunotherapy.
Rights and permissions
About this article
Cite this article
Kalathil, S.G., Thanavala, Y. High immunosuppressive burden in cancer patients: a major hurdle for cancer immunotherapy. Cancer Immunol Immunother 65, 813–819 (2016). https://doi.org/10.1007/s00262-016-1810-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-016-1810-0