Abstract
Immunotherapies like the adoptive transfer of gene-engineered T cells and immune checkpoint inhibitors are novel therapeutic modalities for advanced cancers. However, some patients are refractory or resistant to these therapies, and the mechanisms underlying tumor immune resistance have not been fully elucidated. Immunosuppressive cells such as myeloid-derived suppressive cells, tumor-associated macrophages, tumor-associated neutrophils, regulatory T cells (Tregs), and tumor-associated dendritic cells are critical factors correlated with immune resistance. In addition, cytokines and factors secreted by tumor cells or these immunosuppressive cells also mediate the tumor progression and immune escape of cancers. Thus, targeting these immunosuppressive cells and the related signals is the promising therapy to improve the efficacy of immunotherapies and reverse the immune resistance. However, even with certain success in preclinical studies or in some specific types of cancer, large perspectives are unknown for these immunosuppressive cells, and the related therapies have undesirable outcomes for clinical patients. In this review, we comprehensively summarized the phenotype, function, and potential therapeutic targets of these immunosuppressive cells in the tumor microenvironment.
Similar content being viewed by others
Background
Immunotherapies have achieved rapid development recently and are promising therapeutic options for cancers, especially advanced diseases. These novel therapies include immune checkpoint inhibitors (ICIs), cellular immune therapies like adoptive transfer of engineered T cells, cancer vaccines, and oncolytic virus. However, the immunosuppressive microenvironment mediated or induced by cancers largely limited the efficacy of these novel immunotherapies [1, 2]. Generally, this immunosuppressive microenvironment is composed of cellular and soluble components, promoting tumor progression and favoring the immune escape of cancers [3, 4]. Innate immune cells such as myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), tumor-associated dendritic cells (tDCs), and adoptive immune cells like the regulatory T cells (Tregs) are the main cellular components within the tumor microenvironment (TME). Besides, small molecules such as vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGF-β), and cytokines like interleukin-10 (IL-10) released by cancer cells or immunosuppressive cells are proved to be involved in this process [5,6,7]. In addition, cancer cells also decrease the expression of neoantigens and antigen presentation molecules, such as MHC-I, or upregulate the expression of immune checkpoints on immunosuppressive cells to escape the immune recognition [8, 9]. Thus, therapeutic strategies targeting these immunosuppressive cells include remodeling the TME and increasing the anti-tumor efficacy of immunotherapies. However, even achieved certain success in preclinical studies, some therapies or targets presented limited effectiveness in clinical patients. Thus, in this review, we comprehensively review the current knowledge of the immunosuppressive cells, mainly including MDSCs, TAMs, TANs, Tregs, and DCs in the tumor-associated immunosuppression. We also address the potential strategies to target these immunosuppressive cells for optimizing the therapeutic efficacy in cancer patients.
The immunosuppressive cells in TME
Myeloid-derived suppressive cells (MDSCs)
MDSCs are immature myeloid cells deriving from hematopoietic stem cells residing in the bone marrow. The precursor of MDSCs migrates out of the bone marrow and travels into the extramedullary sites, induced by factors, and becomes the MDSCs. MDSCs are distinctive from neutrophils because they reduce the expression of CD16 and CD62L and increase the expression of Arg-1, CD66B, and CD11b [10]. Currently, several subtypes of MDSCs have been categorized, and each of them has an immunological function. Generally, monocytic MDSCs (M-MDSCs) and polymorphonuclear MDSCs (PMN-MDSCs) are the two main subtypes. M-MDSCs are marked by CD11bhi, Ly6Chi, and Ly6Glo. Instead, PMN-MDSCs are distinguished by a CD11bhi, Ly6Ghi, and Ly6Clo phenotype, while early-stage MDSCs (eMDSCs) display a CD11bhi, CD14−, CD15−, and CD33− phenotype in human [11, 12]. Both M-MDSCs and PMN-MDSCs tend to have an enhanced suppressive phenotype in the TME when compared with conventional MDSCs in peripheral lymphoid organs [13]. M-MDSCs present non-specific suppression mediated by diverse mechanisms such as the secretion of anti-inflammatory and inhibitory cytokines (IL-10, TGF-β, and ROS), the expression of iNOS and Arg-1, and the expression of immune checkpoint inhibitors, and collaboration with other immune cells like Th17 and Tregs. They also produce cytokines and soluble factors that support tumor angiogenesis [14]. PMN-MDSCs are antigen-specific T cell tolerance and non-specific suppression and produce cytokines supporting the angiogenesis in the tumor. eMDSCs are present in peripheral blood as free cells and the TME as enriched cell populations.
Factors responsible for the presentation of MDSCs in TME can be classified into three groups: growth factors including G-CSF, M-CSF, and GM-CSF that are essential for the expansion of MDSCs; cytokines including IL-1 families, IL-4, IL-6, IL-13, and TNF that are inducing the functional maturation of MDSCs; and chemo-attractants including IL-8, CCL2, and CXCL12 for mobilization of MDSCs into TME [15, 16]. After presenting in the TME, MDSCs modulated the interactions between immune cells and the cancer cells, resulting in immunosuppression [17]. Besides, the epithelial–mesenchymal transition (EMT) transcriptional factors, including Snail and Twist1, attracted the immunosuppressive cells such as MDSCs and promoted the expression of immunosuppressive checkpoints, leading to the immunosuppressive TME. In turn, immunosuppressive factors induced tumor cells' EMT, which further promoted the tumor progression [18]. MDSCs are crucial for the pre-metastatic niche formation, which can persist in distant organs (such as the lung) for up to 2 weeks after primary surgeries. These persistent postsurgical MDSCs in the lung showed more robust immunosuppression function than presurgical MDSCs [19, 20]. Overall, due to their critical roles in mediating immunosuppression, MDSCs are regarded as potential targets or biomarkers for immunotherapies [21].
In the clinic, the increase in MDSCs related to tumor progression, attenuated effectiveness of immunotherapies, and poorer outcomes [22, 23]. For example, the results of a whole blood nine colors and 11-parameter flow cytometric assay demonstrated significantly higher levels of MDSC among patients with hepatocellular carcinoma than age-matched healthy controls [24]. Thus, detection of the peripheral blood M-MDSC may be used as a prognostic marker in the clinic [25]. The blood concentration of MDSCs could be good prognostic markers for overall survival after 2 years in refractory diffuse large B-cell lymphoma [21]. In addition, tumors responding to ICIs therapy tend to have higher CD8+ T cells and fewer Gr-1+CD11b+ MDSCs at the early stage following therapy initiation in the mouse model [26]. Furthermore, in patients with different cancer types who received ICIs, the circulating and tumor-infiltrating myeloid populations can be used as predictive biomarkers, both at baseline and on treatment [27].
Tumor-associated macrophages (TAMs)
TAMs are proposed to be the most abundant cell type in TME, orchestrating the immunosuppressive microenvironment [28]. TAMs are usually recruited via chemokines action from the periphery as monocytes and then settled in tumor tissues. Tissue-resident macrophages also migrate to hypoxic or necrotic areas in tumors and then are induced to become TAMs. The number of tumor-infiltrating TAMs is related to poor prognosis in most cancer patients, such as breast cancer, hepatoma, lung cancer, gastric cancer, and other malignancies [29, 30]. Inflammatory M1-macrophages (classically activated) and immunosuppressive M2-macrophages (alternatively activated) are the two main sub-types of macrophages [31]. M1-macrophages are marked with CD11b+F4/80+CD206−, and CD11c+, and M2-macrophages tend to be CD11b+F4/80+CD206+ in mouse model (Table 1) [31, 32]. Different stimulating factors induce the phenotypes of macrophages. For example, LPS is essential for M1-macrophages; IL-4, IL-10, IL-13 for M2a-macrophages; TLR agonists for M2b-macrophages; TNFα, glucocorticoids for M2c-macrophages; and TLR and adenosine A2A receptors for M2d-macrophages [33]. More markers are utilized for the phenotype of macrophages [17]. Besides, the functional mechanisms of each type of macrophage are different. M1-macrophages prompted the destruction of tumor cells, recruited tumor-killing leukocytes, and engulfed tumor cells by producing the immune-killing molecules such as ROS and nitrogen and inflammatory cytokines such as IL-6 and TNF-α. However, M2-macrophages are demonstrated to express co-inhibitory molecules, such as PD-L1, and release anti-inflammatory cytokines, such as IL-10 [34]. In addition, M2-type TAMs enhanced the tumor progression and orchestrated the tumor development and metastasis by releasing matrix metalloproteinases (MMPs), raking down the basement membrane, and remodeling the epithelial cell movements, inducing angiogenesis by releasing VEGF, and recruiting Tregs and MDSCs [28, 35]. Based on this mechanism, dual inhibition of TAMs and PMN-MDSCs is proved to potentiate the efficacy of ICIs [36].
Tumor-associated neutrophils (TANs)
The differentiation of granulocyte-monocyte progenitors into neutrophils is associated with mediators expressed by G-CSF and GM-CSF and starts with the formation of myeloblasts. Then, the myeloblasts differentiate into promyelocytes. The promyelocytes subsequently produce the myelocytes, metamyelocytes, and mature neutrophils [37, 38]. The phenotype of neutrophils in TME depends on the type and stage of cancer. Neutrophils are pro-inflammatory in the early stage of tumor initiation, while they adapt to be an immunosuppressive type as the tumor progresses [17, 39,40,41]. TANs have N1 (anti-tumor) and N2 (tumor-promoting) phenotypes: N1-TANs are marked by CD11b+Ly6G+CD54+CD16+CD170low and CD177+ in mice; However, N2-TANs is characterized by CD11b+Ly6G+PD-L1+CD170high. Another classification strategy is based on the expression of selected molecules, including NI, NM, NA, and NISG, referring to the immature neutrophils, mature neutrophils, aged neutrophils, and neutrophils with interferon-stimulated gene signatures, respectively (Table 1) [37].
TANs suppress the T cells' activation and promote genetic instability, angiogenesis, and tumor metastasis [37, 42]. For mechanisms, TANs inhibit the activation of T cells through secreting the Arg-1, ROS, and NO induced by G-CSF and TGF-β [12, 43]. Besides, TANs promote the proliferation of tumor cells via the production of growth factors like EGF, HGF, and PDGF [44, 45]. The release of neutrophil extracellular traps (NETs) containing HMGB1, neutrophil elastase, myeloperoxidase, and matrix metalloproteinases (MMP8/9) and activating the integrin signaling also promotes the proliferation of tumor cells [46]. NETs are comprised of MMPs, cathepsin G neutrophil elastase, and myeloperoxidase which induces the production of pro-inflammatory cytokines and orchestrates the TME, thereby enhancing tumor progression and metastasis [47, 48]. Prostaglandin E2 (PGE2), expressed by infiltrating neutrophils, promoted tumor proliferation via regulating the inflammation-related gene expression in a self-amplification manner [49]. Besides, TANs also promote tumor angiogenesis by releasing the pro-angiogenic factors BV8, S100A8/9, and MMPs that activate VEGFA in the extracellular matrix [37, 50]. Neutrophil-derived oncostatin M induced the VEGF from cancer cells and increased the detachment and invasive activity of cancer cells [51]. Inhibition of TGF-β signals drove neutrophils to switch to an N1 phenotype with anti-tumor properties. In a TGF-β-rich microenvironment, neutrophils typically have N2 profiles with properties promoting tumor progression and immunosuppressive function [37].
Regulatory T cells (Tregs)
Tregs universally labeled by CD4+CD25+Foxp3+CD127low/− [52,53,54] are differentiated from traditional T cells and divided into two sub-groups, including naturally occurring Tregs (nTregs) and induced-to-adjust T cells (iTregs). These two types of Tregs commonly express the classic marker, Foxp3 [53, 55, 56]. nTregs naturally expand in the normal thymus, and they acquire an inhibitory effect via intercellular interaction. Based on this evidence, nTregs are also called thymus-derived Tregs (tTregs). nTregs regulate the inflammatory response and preserve normal immune tolerance, which can be activated and maintained by NF-κB [57]. iTregs are supposed to be differentiated from peripheral naive T cells induced by factors in TME, including the tumor antigens, inhibitory cytokines such as TGF-β, and other soluble molecules. iTregs are also named peripherally induced Tregs (pTregs) in vivo. iTregs inhibit the anti-tumor immune response of effector T cells, NK cells, and DCs, resulting in tumor progression [53]. There is a new strategy for Tregs classification in which Th-like Tregs subsets were characterized to elaborate their tissue distribution and biological function. For more details, Th1-like Tregs are marked as T-bet+IFN-γ+Foxp3+, Th2-like Tregs are labeled with Gata3+IRF4+IL-4+Foxp3+, and Th17-like Tregs are marked as IL-17+RORγt+Foxp3+ [53]. Th2-like Tregs expressed Gata3 and IRF4 and secret the cytokines IL-4 and IL-13. Th2-like Tregs were induced by IL-4R signaling, which promoted the Gata3 expression [58]. Th17-like Tregs are supposed to express Foxp3 and RORγt, which can be differentiated in the periphery from conventional T cells that retain the suppressive function [59].
Tregs are a double-edged sword by regulating immune homeostasis and inhibiting cancer immune responses. IFN-γ secreted by Tregs enhanced the efficacy of ICIs [60]. The intra-tumoral suppressing T effector cells contributed to the cancer progression that is associated with poor prognosis [61, 62]. Tregs secreted inhibitory cytokines, including IL-10 and TGF-β, thereby inhibiting the immune function [63]. For example, Tregs inhibited the function of CD8+ T cells and DCs through membrane-bound TGF-β [64, 65]. In addition, Tregs eliminated the effector cells by granzymes and perforin, which mediate the cytotoxicity of T lymphocytes and NK cells [53, 66]. Tregs also play essential roles in the angiogenesis through VEGF/VEGFR pathway. It reported that IL-2 on Tregs regulates the effector CD8+ T cells, and the loss of IL-2 would partly explain the exhausted phenotype occurring during tumor responses. This evidence suggests that Tregs influence the function of effector cells by interfering with cell metabolism [67].
Tumor-associated dendritic cells (tDCs)
DCs are differentiated from hematopoietic stem cells, which are resident in the bone marrow. Tumor-inducing signals prevent the production of mature, immunologically active DCs and, at the same time, transform tumor-associated DCs (tDCs) from immunostimulatory cell types to immunosuppressive cell types [68, 69]. This immunosuppressive tDCs included diverse subtypes because of their high heterogeneity, including plasmacytoid DCs (pDCs), conventional DCs (cDCs), and monocyte-derived inflammatory DCs (moDCs) marked by different immune markers (Table 1) [68]. tDCs suppress the activation of cytotoxic T cells and promote the generation of immunosuppressive Tregs, which further regulate the immunostimulatory phenotype at an early stage of tumor progression to an immunosuppressive phenotype [70, 71]. tDCs inhibit the excitation of cytotoxic CD8+ T cells by expressing or secreting immune inhibitory molecules such as PD-L1, T cell immunoglobulin and mucin domain 3 (TIM-3), VEGF, IL-10, PGE-2, and TGF-β [72]. In addition, DCs also promoted the instability of the genome and the angiogenesis to support tumor plasticity [73, 74]. pDCs enhance immunosuppression by promoting the secretion of IL-10 from CD4+Foxp3− T cells [75]. cDCs stimulated the Th-2, Th-17, and T regulatory responses [76]. At last, the moDCs were derived from monocytes and produced pro-inflammatory cytokines, including TNF, IL-12, and IL-6, to promote tumor progression [77].
Other immunosuppressive cells
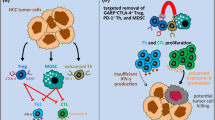
Besides the above immunosuppressive cells, other immune cells existed in TME, mediating the immunosuppressive TME described in recent years. For example, B cells play opposing roles in cancers by suppressing the anti-tumor immunity, of which Bregs are associated with inflammation, autoimmunity, and tumor progression [78, 79]. Bregs negatively modulated the immune responses by releasing anti-inflammatory cytokines, such as IL-10, and expressing co-inhibitory molecules, such as PD-L1 [80, 81]. Another immune cell involved in tumor progression is the tumor-associated mast cell. Mast cells are promoters, bystanders, or guardians of tumors following the cancer stage. Mast cells are essential for the outgrowth of early-stage prostate cancer due to the secretion of MMP9 but become dispensable in advanced-stage [82]. Activated tumor-associated mast cells induced immunosuppression, angiogenesis, tumor invasion, and metastasis by releasing growth factors and proteolytic enzymes [83]. Besides, it has been demonstrated that the number of infiltrating mast cells within the tumor is related to poor prognosis in cancer patients (Fig. 1).
The immunosuppressive cells in tumor micro-environment. Immune cells infiltrate into the tumor microenvironment, interact with each other and tumor cells, and then harbor an immunosuppressive phenotype that is responsible for the immune escape of tumor cells and the following tumor progression. These immunosuppressive cells include MDSCs, M2-macrophages, Tregs, N2-TANs, mast cells, Bregs, dendritic cells. They secrete cytokines like IL-2, IL-10, and TGF-β, growth factors like VEGF, the checkpoints ligands like PD-L1 or express checkpoints on the cell surface like PD-1, TIM-3 on Tregs, that negatively regulate the anti-tumor immune response, remodel the extracellular matrix, and promote the angiogenesis. As a result, these immunosuppressive cells and their interaction generate an immunosuppressive microenvironment and promote the proliferation, evasion, and migration of tumor cells
Targeting immunosuppressive cells in cancers
MDSC-based therapies
Strategies targeting MDSCs include depleting the populations, blocking their migration and recruitment, inhibiting their activity, inhibiting MDSCs metabolism, and promoting the maturation of MDSCs [84]. Here, we summarized the targets involved in the therapeutic strategies based on MDSCs for cancer therapy (Fig. 2).
The potential strategies to target MDSCs. MDSC is the main type of immunosuppressive cell in cancer. Strategies targeting MDSCs to reverse the immunosuppression include depleting the populations of MDSCs by targeting VEGFR and CD33, blocking the migration and recruitment of MDSCs into TME by targeting the CCR2 or CXCR1/2, inhibiting the activity of MDSCs by targeting PGE2 and IDO, promoting the differentiation of MDSCs by TLR agonists, and inhibiting the metabolism of MDSCs by targeting FATP2 and CPT1
Depleting the populations of MDSCs
Low-dose chemotherapeutics, including paclitaxel, cisplatin, 5-fluorouracil (5-FU), and gemcitabine, effectively deplete MDSC populations and enhance anti-tumor immune activity [85,86,87,88]. Moreover, targeting the signals involved in MDSC expansion inhibited the proliferation of MDSCs. Tyrosine kinase inhibitors (TKIs) are extensively studied to eliminate MDSCs. For example, sunitinib prevented the accumulation of MDSCs and restored the T cells' function in renal carcinoma cancer, colorectal cancer, and breast cancer-bearing mice via blockade of VEGF and STAT3 signaling [89]. The inhibitor of Bruton's tyrosine kinase (BTK), ibrutinib, was reported to inhibit the generation and modulate the function of MDSCs in mammary tumors and melanoma to enhance immune-based therapy [90]. Besides, the administration of pazopanib resulted in a dramatic decrease in MDSC and the following enhancement of the PD-1 expressing cytotoxic T and NK effectors (Table 2) [91]. The effect of chemotherapeutics on MDSCs depends on various factors, including chemotherapy doses, dosing schedules, tumor types and stages, and the location of MDSCs. Chemotherapeutics are not specific to MDSCs and affect all rapidly proliferating cells, including anti-tumor T cells. Therefore, the effect of chemotherapy on tumor immunity depends on the balance between immunostimulatory and immunosuppressive effects.
DS-8273a, a TNF-related apoptosis-induced ligand receptor (TRAIL-R2) agonistic antibody, reduced the levels of MDSCs in circulation and decreased tumor-infiltrating MDSCs in advanced cancer patients (NCT02076451) [92, 93]. BI 836858 is a humanized Fc-engineered monoclonal antibody targeting CD33 and depleting the MDSCs through the antibody-dependent cellular cytotoxicity [94]. Besides, the immunotoxin gemtuzumab ozogamicin, a humanized monoclonal antibody against CD33, decreased the level of MDSCs and restored the CAR-T cell effects against cancers [95]. In addition, other researchers have developed a novel peptide‐Fc fusion protein therapeutically targeting the S100A family proteins, thereby depleting MDSCs without affecting the pro-inflammatory immune cells [96]. These strategies are under evaluation in preclinical and clinical studies, and further studies are required to investigate the potential roles in tumors.
Blockade of MDSCs migration and recruitment
The chemokines of CXCR2, like CXCL2 or CXCL5, are essential for PMN-MDSCs recruitment. Targeting the chemokine receptor CXCR2 reduced the MDSCs populations, promoted T cells' infiltration, and improved the efficacy of PD-1 blockade [97, 98]. SX-682, a CXCR1/2 inhibitor, enhanced the T cell-based immunotherapeutic efficacy and benefited patients with MDSC-infiltrated cancers [99, 100]. Besides, CXCR1/2 agonists are the primary mediators of cancer-promoted NETosis [101]. Other CXCR1/2 inhibitors such as reparixin, navarixin, and AZD5069 have also been evaluated in clinical trials to treat patients with advanced cancer [102]. HuMax-IL8 (BMS-986253), a humanized mAbs targeting IL-8, has been assessed to be tolerable when combined with nivolumab in patients with advanced solid tumors in phase I/II study (NCT03400332) [103, 104]. Besides, the combination of CXCR1/2 inhibitors with chemotherapy, anti-angiogenesis drugs, and immunotherapy is also a prospective strategy in cancer therapy.
Another chemokine receptor is CCR5, regulating the migration and recruitment of M-MDSCs into the TME. In addition, MDSCs expressing CCR5 are more immunosuppressive than MDSCs do not express CCR5. Besides, CCR5/CCR5 ligand axis also activates their immunosuppressive functions in the TME, with therapeutic potential [105]. In preclinical studies, silencing of host CCL5 expression in bone marrow delivered by nanoparticles combined with CCR5 inhibitors (Maraviroc) reduced MDSCs and increased anti-tumor immunity [106]. For clinical patients, the blockade of CCR5 inhibits the recruitment and immunosuppressive function of MDSCs, which improves the overall survival of melanoma and breast cancer [105, 107]. In KR158 glioma-bearing animals, the CCR2 antagonist (CCX872) increased the median survival when administrated as monotherapy and further prolonged overall survival when combined with immunotherapy. Further evaluation of the TME indicated that CCX872 treatment decreased tumor-associated MDSCs, elevated tumor-infiltrating lymphocytes, and increased IFN-γ expression [108].
CSF-1/CSF-1R axis promoted the differentiation of MDSCs following the tumor progression [109]. Inhibition of CSF-1/CSF-1R signaling reduced the tumor-infiltrating MDSCs and enhanced the anti-tumor T cell responses [109]. Thus, treatment with CSF-1R inhibitors disrupted the interaction between carcinoma-associated fibroblasts and MDSCs, triggering an increase in the recruitment of granulocytes to the tumors. The CSF-1R inhibitor and CXCR2 antagonist combination blocked tumor granulocyte infiltration and presented strong anti-tumor efficacy [110]. Improved effects are also observed when CSF-1/CSF-1R blockade combines with anti-VEGFR antibodies and ICIs in vivo [111, 112]. Combination therapies based on CSF-1R blockade require further exploration as promising strategies in cancer patients [84].
Other strategies blocking the migration and recruitment of MDSCs include anti-VEGF/VEGFR therapy, anti-S100A8/A9 therapy, and anti-IL-1β therapy in preclinical studies. VEGF is an essential stimulator in the proliferation and recruitment of MDSCs, and the MDSCs, in turn, would promote tumor angiogenesis via secreting cytokines, such as VEGF [113, 114]. Bevacizumab-based therapies significantly reduced the circulating MDSCs levels in patients with solid tumors, especially combining with chemotherapies [115,116,117]. IL-1β promoted tumor progression by inducing inflammation, increasing the E-selectin expression, and driving the migration of MDSCs [118,119,120]. Nucleotide oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome promoted the maturation and secretion of IL-1β. Thus, agents that inhibited the IL-1/IL-1β included IL-1Ra (anakinra), IL-1β antibody (canakinumab), and the inflammasome inhibitor (MCC950) have potential anti-tumor activity in cancers [121, 122]. S100A8/9 are small molecular calcium-binding proteins expressed on MDSCs and involved in tumor progression [123, 124]. Tasquinimod is an oral agent blocking S100A9, reducing the MDSCs infiltration into TME [125, 126]. Currently, several clinical trials have verified that treatment with tasquinimod would improve the free survival of solid cancer patients by reducing the recruitment of MDSCs (NCT01234311, NCT01743469, NCT00560482) [127,128,129].
Inhibiting the activity of MDSCs
Several strategies are reported to reverse the potent immunosuppressive mechanisms of MDSCs to restore the T cell activity and enhance the anti-tumor immunotherapy efficacy. For example, the antisense oligonucleotide STAT3 inhibitor, AZD9150, showed a marked decrease in PMN-MDSCs and is combined with ICIs for diffuse large B‐cell lymphoma (NCT01563302) [130]. Utilizing the TLR9-targeted STAT3 siRNA delivery to block the immunosuppressive function of MDSCs reduced the expression and enzymatic activity of Arg-1, downregulated the STAT3 target genes, and inhibited the activity of T cells [131]. These similar STAT3-dependent mechanisms of MDSCs are also observed in pancreatic cancer [132]. Inhibition of STAT3 potentially enhances CAR-T cells' efficacy administrated in liver metastasis by modulating the MDSCs [133]. In addition, the autocrine of IL-6 mediated activation of the STAT3-DNA methyl transferase axis silenced the TNFα-receptor interacting protein 1 necroptosis pathway that sustained the accumulation of MDSCs. Therefore, targeting IL-6 represents potentially an effective approach to suppressing the survival and accumulation of MDSCs in the TME [134]. JAK-STAT inhibition with ruxolitinib resulted in an anti-tumorigenic TME, characterized by the decreased tumor-promoting cytokines and the reduced infiltrating MDSCs in lung cancer [135].
Phosphodiesterase-5 (PDE5) inhibitors, including sildenafil, vardenafil, and tadalafil, are currently in clinical application for malignant tumors. In tumor mice models, PDE5 inhibition downregulated the Arg-1, and the expression of nitric oxide synthase-2 reduced the recruitment of MDSCs. Besides, the PDE5 inhibition improved the anti-tumor efficacy of adoptive T cells therapy, reversed the tumor-induced immunosuppression, and enabled a favorable anti-tumor immune response that substantially delays tumor progression [136]. In addition, tadalafil reduced the infiltration of MDSCs and Tregs, promoting anti-tumor immunity in patients with head and neck squamous cell carcinoma [137].
PGE2 is crucial for inducing the phenotype and function of MDSCs, whereas the tumor-promoted induction of MDSCs depends on COX2 signaling. The essential role of the COX2-PGE2 pathway in the expansion and persistence of MDSCs highlights the potential function of its management to enhance or suppress immune responses in tumors. Disruption of the COX2-PGE2 axis with COX2 inhibitors suppresses the production of MDSCs-related suppressive factors [138]. In mouse models of glioma, treatment with the COX2 inhibitors, acetylsalicylic acid, or celecoxib inhibited the systemic production of PGE2 and delayed the development of glioma [139]. Besides, targeting the COX2-PGE2 feedback eliminated the self-limiting of type-1 immunity in TME, driven by the synergistic induction of COX2, and enhanced the efficacy of immunotherapies for cancers [140]. The COX2/PGE2 feedback regulates the expression of PD-L1 in tumor-infiltrating myeloid cells. Using COX2 inhibitors to inhibit PGE2 formation reduced PD-L1 expression on TAMs and MDSCs. Therefore, reprogramming the PGE2 metabolism in TME provided a chance to reduce immune suppression [141].
Histone deacetylase inhibitors (HDACs) play essential roles in inducing the differentiation of MDSCs [142]. A combination of low-dose adjuvant epigenetic drugs disrupted the pre-metastatic microenvironment and inhibited the tumor metastases [143]. Low-dose HDAC inhibitor trichostatin-A reshaped the TME and upregulated the expression of PD-L1 by modulating the suppressive activity of TAMs and MDSCs, further enhancing the anti-tumor effects of immunotherapies. Combining low-dose trichostatin-A with anti-PD-L1 significantly enhanced the durable tumor reduction and prolonged the overall survival of tumor-bearing mice [144]. Entinostat enhanced the anti-tumor effect of PD-1 targeting by functionally inhibiting MDSCs and transforming from an immunosuppressive microenvironment [145]. Combining entinostat with anti-PD-1 or anti-CTLA-4 significantly decreased the suppression by PMN-MDSCs, increased in activated granzyme-B producing CD8+ T cells, and improved the tumor-free survival in both breast cancer and pancreatic cancer [146]. Therefore, further research is required to explore the mechanism of combining immunotherapy with HDAC and to develop effective therapies for cancer patients.
Other potential strategies inhibiting the function of MDSCs are also explored and evaluated. For example, the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway was a potential therapeutic target in the tumor, the activation of which cleared the ROS in MDSCs and decreased the risk of tumor metastasis [147, 148]. Besides, the synthetic triterpenoid CDDO-Me inhibited the immunosuppressive activity of MDSCs via activating Nrf2 and inhibiting the generation of ROS in MDSCs (NCT00529113) [149]. In addition, the NOV-002, a glutathione disulfide mimetic, induced the S-glutathionylation and inhibited the production of ROS in MDSCs (NCT00499122) [150, 151].
Promoting the differentiation and maturation of MDSCs
All‐trans‐retinoic acid (ATRA) is supposed to induce the differentiation of MDSCs into mature myeloid cells. ATRA induced the differentiation of MDSCs primarily through the neutralization of high ROS generation, involving a specific increase in the glutathione synthase and the accumulation of glutathione [152]. ATRA treatment blocked the anti-angiogenic therapy-induced expansion of MDSC. Concomitant therapy with ATRA holds the potential to improve anti-angiogenic therapy in breast cancer [153]. Due to the function of modulating the differentiation of MDSCs, the combinational therapies of ATRA and immunotherapies have synergistic anti-tumor efficacy. For example, ATRA combined with ipilimumab significantly reduced the number of MDSCs in peripheral circulation compared with ipilimumab monotherapy in patients with metastatic melanoma (NCT02403778) [154]. In addition, combinational therapy utilizing GD2-CAR T cells and ATRA significantly improved the anti-tumor efficacy against sarcoma in a mouse model. Retinoids can reduce the suppressive function of MDSCs, while co-administration of retinoids can enhance the effectiveness of CAR T cells therapy in solid tumors [155]. The combination of anti-PD-1 therapy with ATRA improved the proliferation of local and systemic T cells and produced tumor-specific immunity. Accumulation of MDSCs in LKB1-deficient NSCLC can be overcome via the administration of ATRA, sensitizing the tumors to immunotherapy [156]. Overall, ATRA is an effective strategy in anti-tumor therapies, while off-target effects may contribute to diminished therapeutic efficacy. ATRA can enhance immune response via inducing the differentiation of MDSCs, especially in combination with other anti-tumor strategies.
The increased activity of casein kinase 2 (CK2) is responsible for the Notch phosphorylation and downregulation. Utilizing siRNA or pharmacological inhibitors to inhibit CK2 restored the Notch signaling in myeloid cells and significantly improved their differentiation [157]. Besides, inhibition of CK2 substantially reduced the amount of PMN-MDSCs by a block of differentiation. These modulatory effects of CK2 inhibitors on myeloid cell differentiation in the TME would be synergized with the anti-tumor effects of ICIs [158].
Other potential therapies included promoting the differentiation and maturation of MDSCs. For example, the TLR7/8 agonists enhanced the anti-tumor efficacy by promoting the differentiation of MDSCs into macrophages and DCs (NCT02124850) [159, 160]. Besides, polyinosinic–polycytidylic acid, namely poly I: C, which is a synthetic double-stranded RNA ligand for TLR3, has shown promising potential in decreasing the MSDCs and abrogating their immunosuppressive functions, especially when combined with irradiation, vaccines, and CAR-T therapy [161,162,163]. Finally, the traditional Chinese medicine saposhnikovia root divaricate prim-o-glucosylcimifugin was identified as an inhibitor of PMN-MDSCs. The prim-o-glucosylcimifugin exhibited a favorable synergistic anti-tumor efficacy with a PD-1 inhibitor [164]. Also, the administration of β-glucans, curdlan, curcumin, and icariin accelerated the differentiation of MDSCs and weakened the related immunosuppression in preclinical tumor models [165,166,167,168]. Nevertheless, these strategies in cancer patients require further investigation.
Inhibiting MDSCs metabolism
Transcription factors liver-X nuclear receptors (LXRβ and LXRα) are associated with the fatty acid, cholesterol, glucose, and lipid metabolism. These receptors are potential targets for regulating the metabolism of MDSCs. For example, LXR agonists, GW3965 and RGX-104, reduced the accumulation of MDSCs in mice models. In a phase I trial, patients were treated with first-in-human dose-escalation by targeting the LXR/apolipoprotein E axis to regulate innate immune suppression and activation of cytotoxic T lymphocyte (CTL) responses [169]. LXR agonist upregulated the apolipoprotein E (ApoE), which binding to the low-density lipoprotein receptor-related protein 8 (LRP8) expressed on MDSCs, leading to the depletion of MDSCs [169, 170]. Another fatty acid-related target is carnitine palmitoyltransferase 1 (CPT1, a rate-limiting enzyme in the fatty acid pathway). Etomoxir, a CPT1 inhibitor, significantly abrogated the immunosuppressive function of MDSCs and delayed the tumor growth when combined with low-dose chemotherapy [171]. Lipofermata, a fatty acid transport protein 2 (FATP2) inhibitor, restrained the activity of PMN-MSDCs and restricted the tumor progression through the STAT5 pathway alone or combined with ICIs [172, 173].
Glycolysis and the related pathways are potential targets to modulate the immunosuppressive activity of tumor-infiltrating MDSCs. The glycolysis of MDSCs in cancers is modulated by HIF-1α and AMP-activated protein kinase (AMPK) [174, 175]. Metformin activated the AMPK, thereby inhibiting the immunosuppressive function of MDSCs and delaying the tumor progression by inhibiting immune-related NF-κB and JAK-STAT pathways [174, 176, 177].
Indoleamine 2,3-dioxygenase (IDO) is a critical enzyme in the tryptophan–kynurenine–aryl hydrocarbon receptor axis. IDO is over-expressed in tumor-infiltrating immune cells and tumor cells, facilitating the recruitment and activation of MDSCs [178,179,180]. The cytokine IL-6 triggered the IDO promotor in MDSCs through the STAT3 pathway [181]. A phase III study involving the IDO inhibitor, epacadostat, combined with pembrolizumab, has comparable outcomes between the epacadostat group and placebo group in patients with advanced melanoma (NCT02752074) [182]. Recently, another phase II trial of indoximod showed that the addition of indoximod to a taxane did not improve the PFS compared with a taxane alone in patients with ERBB2-negative metastatic breast cancer (NCT01792050) [183]. Finally, novel inhibitors targeting the Trp-Kyn-AhR pathways, such as navoximod, tryptophan mimetics, kynurenine-degrading enzymes, and aryl hydrocarbon receptor antagonists, are also explored in cancer therapy based on MDSCs [182, 184,185,186].
The TAMs-based targeted therapies
Strategies targeting TAMs include depleting macrophages and blocking the recruitment of TAMs, targeting the activation of TAMs, modulating the phagocytosis of TAMs, and chimeric antigen receptor macrophage cell therapy. Here, we concluded the relevant therapeutic strategies on TAMs for cancer therapy (Fig. 3).
The potential targets of TAMs in cancer therapy. M2-type TAMs in TME mediate the immunosuppression and promote the growth of tumor cells, as well as the resistance of cancer to immunotherapy. Strategies targeting the TAMs to reverse the immunosuppression include depleting and blocking the recruitment of TAMs into TME by targeting the CCR2 or CXCR1/2, targeting the activation of TAMs by CD40 and TLR7/8, modulating the phagocytosis of TAMs by targeting SIRPα, LILRB1, and Siglec-10. Furthermore, with the development of adoptive cell therapy, CAR-M represents a novel strategy that applies modified macrophages by adding specific CAR to them, which enhances the phagocytosis of macrophages on tumor cells. Besides, other advantages of CAR-M are identified, such as the resistance of CAR-M being polarized to M2 type
Depleting TAMs and blocking the recruitment of TAMs
CSF-1R is expressed on macrophages and their frontier monocytes, which is an important signal for the differentiation of TAMs. Nanoparticles loaded with anti-CSF-1R siRNA specifically depleted the M2-TAMs from melanoma and then restored the immune function of T cells, decreased the tumor size, and prolonged the overall survival [187]. 3D185 is a novel double inhibitor targeting both the FGFRs and CSF-1R. This inhibitor inhibited the polarization of macrophages to M2-like and the tumor progression by remodeling the tumor microenvironment in TAM-dominated murine models [188]. PLX3397, also named pexidartinib, is a CSF-1R kinase inhibitor that is supposed to reduce the numbers of TAMs effectively and the angiogenesis and ascites in mouse models with mesothelioma. PLX3397 combined with DC vaccination synergistically enhanced the overall survival with decreased TAMs and increased the number and function of CD8+ T cells [189]. A clinical trial suggested that emactuzumab, a monoclonal antibody against CSF-1R, combined with paclitaxel, depletes immunosuppressive TAMs in patients with advanced solid tumors [190]. In addition, CSF-1R inhibition altered the polarization of macrophages via secreting GM-CSF and IFN-γ, which blocked glioma progression in a mouse model [191]. Furthermore, targeting TAMs using a CSF-1R inhibitor combined with radiotherapy enhanced the survival of glioma in preclinical models (Table 3) [192]. These therapeutic strategies are not necessarily effective because CSF-1/CSF1R blockade may increase the activity of Tregs in the TME and compensate by signaling through other pro-survival pathways.
CCL2/CCR2 axis is associated with several types of immunosuppressive cell recruiting, including TAMs, MDSCs, and Tregs [193,194,195]. Targeting TAMs by inhibiting CCR2 decreased the tumor-initiating cells, resulting in metastasis inhibition and enhancement of the anti-tumor T cell responses in pancreatic tumor and liver cancer [196,197,198]. Dual targeting of the CCR2+ TAMs and CXCR2+ TANs improved the anti-tumor immunity in pancreatic adenocarcinoma [199]. CCL2/CCR2 inhibitors, while having some success in preclinical models, have not demonstrated similar levels of efficacy in clinical trials. CCL2/CCR2 blockade has little effect on established TAMs and still promotes tumor progression. Besides, upregulation of stromal cell-derived factor 1 alpha (SDF-1α/CXCL12) induced by hypoxia was supposed to contribute to the recruitment of TAMs [200, 201]. AMD3100, a CXCR4 antagonist, prevented the polarization of TAMs toward an immunosuppressive phenotype after the administration of sorafenib, which inhibited tumor growth and reduced the lung metastases in mice models [202]. Besides, inhibiting CXCR4 combined with anti-angiogenesis drugs improved the anti-tumor efficacy via the ERK pathway in liver cancer [203].
Bisphosphonates depleted TAMs that suppressed the angiogenesis, normalized the tumor vasculatures, and relieved tumor hypoxia, which offered favorable synergistic therapeutic efficacy [204,205,206]. Nanoparticles containing zoledronic acid caused a significant reduction of the TAMs, inducing the complete remission and increasing the survival of tumor xenografts [207]. The same preclinical outcome was observed in the S180 tumor-bearing mice model administrated with Bletilla striata polysaccharide and alendronate conjugate [208]. Yondelis, also named Trabectedin, is a novel therapeutic agent extracted from the tunicate Ecteinascidia turbinate, resulting in inhibition of the viability and function of TAMs [209]. Renalase is a secreted flavoprotein that increased markedly in TAMs, promoting melanoma progression. Therefore, targeting renalase has potential therapeutic implications for the management of melanoma [210]. Besides, the accumulation of TAMs is related to the expression of integrin αvβ3 on tumor cells, which are known drivers of epithelial tumor progression and drug resistance. LM609 is a monoclonal antibody targeting αvβ3, and this agent engages the macrophages to induce cellular cytotoxicities on αvβ3-expressing tumor cells [211].
Targeting the activation of TAMs
Reprogramming TAMs toward the M1 phenotype would prevent immunosuppression and improve anti-tumor immunity. The selective depletion of the microRNA processing enzyme DICER in TAMs promoted M1-like macrophages programming, characterized by overactive IFN-γ/STAT1 signaling. DICER/Let-7 activity against IFN-γ-induced immunostimulatory M1-like TAM activation has potential therapeutic significance [212]. Agonistic anti-murine TNF receptor such as CP-870893, a fully human anti-CD40 monoclonal antibody, has been assessed in clinical trials with beneficial efficacy in melanoma and pancreatic cancers [213, 214]. The combination therapy of agonistic anti-CD40 monoclonal antibody and CSF-1R inhibitor effectively inhibits tumor growth in mouse models of melanoma by targeting TAMs, transforming “cold” into “inflamed” tumor microenvironment [215]. TAM programming with agonistic anti-CD40 increased both Ly6Chigh TAMs and the intra-tumoral accumulation of TCR-engineered T cells, which finally increased tumor cell apoptosis [216].
TAM activation inhibited the TLR-mediated M1-type macrophage polarization. Treatment with resiquimod, a TLR7/8 agonist, doubled the overall survival in mice models [217]. R848-Ad, an adamantane-modified derivative of resiquimod, enables TAM-targeted drug delivery and then enhances the anti-tumor effects in a mice colon cancer model [218]. In addition, targeted delivery of the long peptide antigen to TAMs through nanohydrogels with a TLR agonist activated the TAMs, inducing antigen presentation activity and then transforming resistant tumors into susceptibility to adoptive transfer of tumor-specific T cell receptor engineered T cells [219]. Combining the TLR agonists with focal ablation drives a highly effective immune response [220].
The PI3K/AKT pathway sustains the recruitment of inflammatory cells and is essential for tumor invasion. LY294002, a PI3Kα inhibitor, blocked the recruitment of TAMs, in addition to inhibit the TAM-mediated tumor invasion [221]. Another PI3Kα inhibitor, alpelisib (BYL719), had favorable anti-tumor activity in head and neck squamous cell carcinoma. But the TYRO3 and AXL receptors meditated the resistance to alpelisib in this model [222]. Combined treatment with PI3Kα and either EGFR, AXL, or PKC inhibitors reverted this resistance [223, 224]. In addition, PIK3CA mutations predicted the response to PI3K inhibitors in lung cancer. The combination of PI3K inhibitor and CDK4/6 inhibition enhanced the anti-tumor response to the two single agents [225].
Modulating the phagocytosis of TAMs
Signal regulatory protein alpha (SIRPα) on TAMs interacts with CD47, a “don't eat me” signal on cancer cells, preventing the phagocytosis of macrophages for cancer cells [226]. Thus, disruption of the SIRPα-CD47 axis would protect effectively against solid tumors by inducing tumor phagocytosis [227,228,229]. In pancreatic cancers, targeting CD47 efficiently enhanced the phagocytosis of tumor cells and the apoptosis of macrophages [230]. Efficiently blockade of the CD47-SIRPα pathway with genetically engineered cell-membrane-coated magnetic nanoparticles promoted the repolarization of M2-TAMs, triggering the potent immune responses in melanoma and breast cancer models [231, 232]. Currently, the anti-CD47 monoclonal antibodies, including Hu5F9-G4, CC-9002, TI-061, and SRF231, are under clinical trial evaluation [233]. CD47/SIRPα blockade cannot induce phagocytosis on its own, which should be used in combination with an opsonizing agent.
CD24 is suggested as a novel “don't eat me” signal that promotes the tumor immune escape [234, 235].CD24 is a dominant innate immune checkpoint regarding as a promising immunotherapy target for cancer. CD24 interacting with the inhibitory receptor sialic-acid-binding Ig-like lectin 10 (Siglec-10) on TAMs promoted the evasion of the tumor. Gene ablation of CD24 or Siglec-10 and monoclonal antibodies to block the CD24-Siglec-10 interaction enhanced the phagocytosis of CD24-expressing tumors, resulting in a decrease in macrophage-dependent tumor growth [236, 237].
MHC-I controls the phagocytic function of macrophages, mediated by the inhibitory receptor LILRB1 on TAMs. Disruption of MHC-I or LILRB1 potentiated the macrophages' phagocytosis of cancer cells, which defined the MHCI-LILRB1 pathway axis as an “eat me/don't eat me” signal [238]. Antibody-dependent cellular phagocytosis (ADCP) is an essential mechanism for antibody tumor therapy based on TAMs. Resiquimod, an immune-activating agent, reeducated the TAMs from M2 to M1, boosting the antibody-dependent cellular phagocytosis and enhancing the anti-tumor efficacy [239].
Chimeric antigen receptor macrophage (CAR-M) cell therapy
The adoptive cell therapies with chimeric antigen receptor (CAR) technology are getting more and more attention due to the reported efficacy in targeted cancer phagocytosis [240]. CAR-T cell therapy has demonstrated promising efficacy in hematologic malignancies, while its potential application in solid tumors is challenging [240]. Researchers are investigating other immune cells as possible CAR platforms to deal with the limitations of CAR T cells [241, 242]. CAR expression in macrophages plays an essential role in enhancing phagocytosis, polarizing the M2 phenotype to the M1 phenotype, and stimulating the anti-tumor activity of T cells. Folate receptor β (FRβ) is exclusively expressed on TAMs, which is associated with poor clinical outcomes in patients with solid tumors [243]. Conditionally depleting M2-type macrophages with CAR-T cells to target FRβ successfully eliminated the FRβ+ M2-TAMs and induced an influx of tumor-specific CD8+ T cells [244]. Anti-CD123 CAR-T cells are supposed to provide dual targets for the CD123+ tumor cells, and the CD123+ M2-type macrophages in the tumor microenvironment efficiently eradicate the Hodgkin’s lymphoma [245].
Morrissey et al. designed new CAR structures named chimeric antigen receptors for phagocytosis (CAR-P). They screened kids of phagocytic receptors in mice, such as FcRɣ, MerTK, and Bai1, and utilized these receptors as the intracellular signaling domains of the CARs. As a result, the CAR-P promoted the phagocytosis of tumor cells and decreased the tumor cell count by over 40% when combined with the Raji B tumor cell line [246]. The anti-CD19 CAR encodes for the intracellular domain of CD3ζ, which is a part of the T cell antigen receptor complex, a type 1 transmembrane protein that forms a homodimer and is involved in ADCP [247].
Other designs of CAR-Ms included tailoring CAR-Ms of target tumors with HER2 overexpression. These CAR-Ms activated the CD147 signaling, thereby inducing matrix metalloproteinases to undermine the extracellular matrix of breast cancer and significantly inhibit tumor growth [248]. CAR-Ms against HER2 lead to a significant decrease in metastatic tumor burden in ovarian cancer. Therefore, CAR-M decreased tumor burden and extended the overall survival in murine models, converted bystander M2 macrophages into M1-type, and boosted anti-tumor T cell activity [240]. CT-0508 has acquired Food and Drug Administration approval as an anti-HER2-CAR-M from CARISMA Therapeutics. The phase I clinical trial of CT-0508 against HER2 positive solid tumors has launched (NCT04660929). Another strategy of CAR-Ms was targeting the CCR7+ cells, which were important in inducing cancer cells metastasis to lymphoid organs. CAR-Ms with CCR7 targeting significantly reduced the tumor growth and prolonged the overall survival in a mouse model [249]. Moreover, CAR-Ms generated from induced pluripotent stem cells were capable of activating phagocytosis and reducing the tumor growth in ovarian and pancreatic cancer [250]. The key to the successful clinical use of cell therapy is its scalability and reproducibility. However, CAR-M therapy requires the extraction of the patient's blood and a 1-week manufacturing process, which may be the drawbacks that limit its widespread application.
TANs-related therapies
Strategies targeting TANs include depleting TANs and inhibiting their recruitment, inhibiting the functions of TANs, blocking the development of TANs, and reprogramming TANs. We generalized the targeting strategies on TANs for cancer therapy (Fig. 4).
The currently proposed therapy based on TANs targeting. Neutrophil is the first responder to injury. However, the roles and importance of neutrophils in inducing the tumor progression as well as the generation of immunosuppressive microenvironment have been revealed recently. Strategies targeting the TANs to reverse the immunosuppression include depleting and blocking the recruitment of TANs into TME by targeting the CXCR1/2, CXCR4, and CSF-1R, inhibiting the functions of TANs by targeting VEGFR and PGE2, blocking the development of TANs by IL-17/IL-23 inhibitors, and reprogramming TANs by targeting TGF-β, NAMPT, and FATP2
Depleting TANs and inhibiting their recruitment
Chemokines CXCL1, 2, 5, 6, 8 and their receptors CXCR1/2 are co-opted by multiple cancers to facilitate the recruitment of neutrophils [251, 252]. A systemic accumulation in CXCR2+ TANs correlates with an unfavorable prognosis in patients with pancreatic cancers. CXCR2 blockade prevented the mobilization of neutrophils from circulation and augmented the efficacy of chemotherapies [199]. Thus, the CXCR1/2 inhibitors, SX-682, and navarixin inhibited the extension of TANs and increased the effectiveness of immunotherapy in a mouse model [99].
CXCR4 also contributed to neutrophil recruitment in the tumor metastasis [253, 254]. Deleting CXCR4 in myeloid cells enhanced the anti-tumor immune response, resulting in significantly reduced tumor growth in melanoma [255]. Plerixafor is a CXCR4 antagonist that augments the frequency of circulating neutrophils by releasing them from the marginal pool present in the lungs while preventing neutrophils from returning to the bone marrow. These interactions implicated an essential role of CXCR4-CXCL12 in regulating neutrophil margination in the lungs [256]. Combination therapy with BL-8040/BKT140, a CXCR4 inhibitor, and the pembrolizumab in patients with pancreatic cancer reduced the infiltration of TANs and increased the function of cytotoxic T cells (NCT02826486) [257].
IL-17 cytokine family members, IL-17A and IL-17C, contribute to the recruitment of neutrophils in solid tumors [258,259,260]. Inhibition of the IL-23 and IL-17 axis decreased the amounts of neutrophils mediated by G-CSF [261, 262]. Hence, inhibition of G-CSF would reduce the number of neutrophils and show anti-tumor efficacy in preclinical tumor models [263]. In addition, the complement component 5-a (C5a) in the TME is also an effective attractant for neutrophils [264, 265]. IPH5401, an anti-C5aR antagonist antibody, is currently being evaluated combined with durvalumab (NCT03665129). Moreover, tumor-derived oxysterols recruit TANs which is dependent on the CXCR2 pathway, thereby facilitating tumor growth by promoting angiogenesis and immunosuppression. Distracting with the oxysterol-CXCR2 pathway delayed the tumor growth and prolonged the overall survival in the tumor-bearing mice model [266].
Neutrophils also expressed FcγRs and eliminated the cancer cells via the antibody-dependent cellular cytotoxicity (ADCC). The depletion of neutrophils directly leads to a reduction in the efficacy of monoclonal antibodies against CD52 (alemtuzumab) or CD20 (rituximab) [267]. Neutrophils in humans are supposed to express FcαRI, also called CD89, a high-affinity receptor for IgA and an inducer of ADCC [268]. FcγR plays an essential role in monoclonal antibody-related anti-tumor therapy. The identity of FcγR-bearing cells that provide cytotoxic activity in tumor patients and the extent to which monoclonal antibodies can manipulate neutrophils remain unknown. The IgA-mediated cytotoxic activity of neutrophils was enhanced after the blockade of the CD47-SIRPα axis [269, 270]. Targeting the CD47-SIRPα axis combined with other therapeutic antibodies increased the anti-tumor efficacy of neutrophils [271, 272].
Neutrophils highly express the ligands of immune checkpoints, including V-domain immunoglobulin suppressor of T cell activation (VISTA) and PD-L1 [273,274,275]. Blocking VISTA turned the DCs and monocytes into pro-inflammatory cells that promote the activation of T cell-mediated anti-tumor immunity [274]. Blockade of PD-L1 on neutrophils enhanced their cytotoxic activity against tumor cells [276, 277]. Moreover, other checkpoints, such as CD200R, paired immunoglobulin-like type 2 receptor alpha (PILRα), atypical chemokine receptor 2 (ACKR2), leukocyte immunoglobulin-like receptor B2 (LILRB2), and SIRPα, also highly expressed on neutrophils [278,279,280,281,282]. Therefore, depleting TANs and inhibiting their recruitment are an effective preventive approach to prophylactic the immunosuppressive activity of the tumor microenvironment, which may improve the efficacy of immunotherapy.
Inhibiting the functions of TANs
Preclinical evidence indicated that the overexpression of COX2 in tumors promotes immune evasion through the production of PGE2. COX inhibition and immune checkpoint inhibitors synergistically promoted the activation of anti-tumor T cells. For advanced melanoma patients, the median time to progression was significantly increased in ICI plus COX inhibition (COXi) versus ICI monotherapy. For patients with NSCLC, ICI plus COXi is also related to prolonged median time to progression compared with ICI alone. In the melanoma and NSCLC cohorts, patients who received ICI plus COXi had a significantly higher objective response rate at 6 months than those who received ICI alone. Hence, compared with ICI alone, in patients with metastatic melanoma and NSCLC, the simultaneous use of COXi and ICI was significantly associated with a longer median time to progression and improved objective response rate [283]. Collectively, these findings identify that the COX inhibition in combination with ICI is a promising target to increase the anti-tumor activity of neutrophils. Other strategies like PI3K inhibitors, PDE5 inhibitors, RTK inhibitors, and sildenafil for inhibiting TANs functions also had positive anti-tumor efficacy similar to the above therapeutic strategy based on MDSCs.
Blocking the development of TANs
The recognized IL-12 cytokine family consisted of IL-12, IL-23, IL-27, and IL-35, which play crucial roles in developing immune responses in cancers. Targeting this axis may benefit combination immunotherapies in vivo [284, 285]. Tumor-targeted oncolytic adenovirus would deliver non-secreting IL-12 to tumor cells and significantly enhance the survival of animals with pancreatic cancer [286]. Combination therapy with CAR-T cells targeting tumor-specific EGFRvIII and locally delivered doses of IL-12 achieved effective anti-tumor responses in glioblastoma. Furthermore, IL-12 enhanced the cytotoxicity of CAR-T cells, remodeled the TME, augmented the infiltration of pro-inflammatory CD4+ T cells, and reduced the numbers of Tregs [287]. Therapeutic T cells that target tumor antigens are genetically engineered to express membrane-anchored IL-12, which demonstrated limited systemic exposure and improved efficacy in the mouse model [288].
Reprogramming TANs
TGF-β is correlated with a poor prognosis in advanced cancers. Aberrant upregulation of TGF-β expression in the TME promoted tumor progression, metastasis, and resistance to immuno-modulatory therapies [289]. Based on this fundamental, several types of TGF-β inhibitors have been evaluated in clinical trials, such as monoclonal, neutralizing, and bifunctional antibodies; antisense oligonucleotides; TGF-β-related vaccines; and receptor kinase inhibitors [290]. The combination of galunisertib, a TGF-β inhibitor, with PD-L1 blockade led to improved inhibition of tumor growth and complete regressions in the colon cancer mouse model [291]. M7824 (MSB0011359C), a bifunctional fusion protein made up of a monoclonal antibody against PD-L1 and the extracellular domain of human TGF-β receptor II, suppressed the tumor growth and metastasis more efficiently than treatment with either anti-PD-L1 antibody or TGF-β trap monotherapy.
Moreover, M7824 is an efficient combination cooperator for chemotherapy or radiotherapy in murine models [292, 293]. Overall, TGF-β is a neutrophil chemotactic agent. Inhibition of TGF-β may affect the recruitment of TANs. Although several clinical trials related to TGF-β inhibitors, their side effects, including off-target effects and cytotoxicity, should be paid attention to. Further studies are needed to understand how manipulation of TGF-β in tumor patients might affect various neutrophil subtypes.
A recent study proved that hypoxia was a potent determinant of TANs phenotypes. Utilizing a uterine cancer mouse model and the administration of respiratory hyperoxia to improve tumor oxygenation. Relief of tumor hypoxia unleashed the tumoricidal potential of TANs through the production of NADPH oxidase-derived reactive oxygen species and MMP-9 [294]. TANs overexpressed FATP2, which was restrained by GM-CSF through the activation of the STAT5 transcription factor. Selectively pharmacological inhibition of FATP2 revoked the suppressive activity of TANs, mediated by arachidonic acid and PGE2, and then substantially delayed tumor progression [172]. The angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonist attenuated the tumor growth and enhanced the neutrophil hyper-segmentation dependent on the mTOR pathway [295]. Inhibition of nicotinamide phosphoribosyltransferase in TANs led to the anti-tumor conversion of neutrophils and attenuated the tumor angiogenesis and growth in a melanoma mouse model [296].
Tregs-related therapies
Strategies targeting Tregs include depletion of Tregs, inhibiting the functions of Tregs, and targeting the immune checkpoints on Tregs. Here, we summarized the potential targeted strategies on Tregs for cancer therapy.
Depletion of Tregs
Infiltration of Tregs into TME is associated with poor prognosis as the Tregs can diminish anti-tumor immune response. However, conditional depletion of Tregs may concurrently induce the deleterious autoimmunity unless specifically targeting terminally differentiated effector Tregs rather than all Foxp3+ T cells [55, 297, 298]. CD25 is a high-affinity receptor subunit of IL-2 and a selective target for Tregs depletion. Tregs depletion enhanced their capacity to elicit strong CD4+ conventional T cells responses and ensuing anti-tumor protection [299, 300]. The preclinical assessment of an anti-human CD25 (RG6292) antibody with equivalent characteristics demonstrated efficient Tregs depletion without significant immune-related toxicity [301]. CD25 is expressed at high levels on Tregs and is a target for cancer immunotherapy. Anti-CD25 antibodies exhausted the peripheral Tregs, and the upregulation of inhibitory FcγR IIb at the tumor area prevented the depletion of intra-tumoral Tregs, which may be the reason for the lack of anti-tumor activity previously observed in preclinical models. Fc-optimized anti-CD25 antibody effectively depleted the tumor-infiltrating Tregs, increased effector-to-Treg ratios, improved the control of established tumors, and synergized with anti-PD-1 antibodies to eradicate tumors [302]. Systemic administration of anti-CD25 does not completely eliminate Tregs, but it does prevent Tregs function, thereby safely enhancing tumor immunity in a glioma mouse model [303]. Daclizumab, an anti-CD25 antibody, efficiently depleted all Tregs from the peripheral circulation.
Furthermore, the residual daclizumab suppressed de novo induced Tregs during DC vaccinations [304]. Resistance to PD-1 blockade and radiotherapy is mediated by the upregulation of TIM-3 and the infiltration of Tregs. Treatment with anti-TIM-3 concurrently with anti-PD-L1 and radiotherapy delays tumor growth and decreases Tregs. The analysis of relapsed tumors revealed a resurgence of Tregs. Targeted Tregs depletion with anti-CD25 antibody restored the anti-tumor immunity and lead to tumor rejection and the reaction of immunologic memory [305]. Radiotherapy plus Tregs depletion improved the anti-tumor response. Thus, combining radiotherapy and anti-CD25/CTLA-4 monoclonal antibody resulted in decreased Tregs and enhanced anti-tumor immunity, suppressed the tumor growth, and improved the overall survival (Table 4) [306].
Treg highly expressed CCR4 which is associated with a poor prognosis in multiple tumors. CCR4 on activated effector Tregs is functionally related to chemical kinetic migration and is responsible for extending these cells to the tumor area [307]. Depletion of Tregs may provide a basis for conducting a clinical trial to study Tregs elimination by administering anti-hCCR4 monoclonal antibody (KM2760) to patients with solid cancer [308]. CCR4 blockade inhibited the tumor growth and the infiltration of Tregs that prolonged the survival in a bladder cancer model [309]. Pharmacological antagonism of the CCR4 receptor with selective CCR4 antagonist, CCR4-35, effectively inhibited the recruitment of Tregs and resulted in enhanced anti-tumor efficacy, overcoming the immune resistance [310]. Depletion of CCR4+ T cells is equivalent to total removal of Foxp3+ Tregs via CD25+ T cells elimination. Administration of anti-CCR4 monoclonal antibody in vivo markedly decreased the Tregs fraction, augmented the responses of NY-ESO-1 specific CD8+ T cells, and evoked the anti-tumor immune responses [311]. CCL2 is commonly secreted by macrophages and is essential for recruiting CCR4+ Tregs and CCR2+ M-MDSCs. Administration of the small-molecule antagonist of CCR4 improved the median survival in a glioblastoma mouse model [312]. Due to the activity of remodeling the TME by targeting CCR4, the immune combinational therapy based on CCR4 targeting is promising. For example, when combined mogamulizumab, an anti-CCR4 antibody, and nivolumab in patients with advanced solid tumors, four out of 15 hepatocellular carcinoma patients (27%) had confirmed tumor responses, one out of 15 pancreatic cancer patients had confirmed response and two out of 15 pancreatic adenocarcinoma patients had unconfirmed responses. Furthermore, during the treatment, the populations of effector Tregs marked by CD4+CD45RA−FoxP3high reduced, and CD8+ T cells in tumor-infiltrating lymphocytes (TILs) augmented (NCT02476123) [313]. A phase I study showed that depletion of Tregs by KW-0761, a humanized CCR4 antibody, was investigated in patients with solid cancer. In this trial, four of 10 patients show stable disease during treatment. In addition, the administration of KW-0761 resulted in the efficient depletion of Foxp3+ Tregs in the peripheral blood mononuclear cells. Thus, the combination of KW-0761 with other strategies of immunotherapies, including cancer vaccines or ICIs, is a promising approach to enhance the anti-tumor immune responses [314].
Inhibiting the functions of Tregs
The maintenance and function of Tregs are dependent on PI3Kδ signaling. Selectively depletion of Tregs with PI3K inhibitors increased the activity of CD8+ T cells and prevented tumor metastasis [315,316,317]. Besides, impairment of the activation of PI3K pathway through silencing the microRNA-126 in a breast cancer model reduced the induction and suppressive function of Tregs [318]. Moreover, inhibition of the PI3K activity combined with anti-PD-1 and/or anti-CTLA-4 monoclonal antibodies enhanced the therapeutic efficacy in mouse models of head and neck cancer [319]. Therefore, these findings suggested that inhibiting PI3K signaling combined with ICIs can selectively deplete Tregs, reduce the suppressive function of Tregs, and alleviate Treg-mediated resistance.
Tregs secreted cytokine IL-10, which is associated with poor prognosis in cancers, involving the activation of tumor-resident APC and suppression of effective T cell functions [320, 321]. Moreover, IL-10+ and IL35+ Tregs cooperatively limited the anti-tumor immune response by Blimp-1-mediated exhaustion of tumor-infiltrating T cells and upregulation of immune checkpoints, including PD-1, TIM-3, lymphocyte activation gene 3 (LAG-3), T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT) [322]. Furthermore, IL-10 deficiency reduced the Tregs-mediated immunosuppression through reduction of the expression of neuropilin on Tregs, leading to the tumor regression and enhancing the anti-tumor immunity [323, 324]. The pegylated recombinant human IL-10, AM0010 or pegilodecakin, increased the serum levels of IFN-γ and IL-18 and reduced the levels of TGF-β. These alterations of cytokines resulted in activated intra-tumoral CD8+ T cells and induced the objective tumor responses in advanced solid tumors (NCT02009449) [325, 326]. Furthermore, the combination of AM0010 with PD-1 blockade induced the expansion of LAG-3+ PD-1+ CD8+ T cells [326]. Besides, blocking IL-35 increased the number of tumor-infiltrating effective T cells, enhancing the anti-tumor immune response and reducing tumor growth in multiple mouse models [327].
CD39/CD73/Adenosine receptor (A2AR) pathways are potential targets to reduce the induction and suppressive function of Tregs [328,329,330,331]. A2AR stimulation enhanced the Treg-mediated immunosuppressive mechanism functionally. In addition, A2AR-mediated stimulation of lymphocytes using A2AR agonists induced the expansion of Tregs ex vivo [332]. Moreover, CGS21680, an A2AR agonist, upregulated the expression of CD39 and CD73 through E2F transcription factor 1 and cyclic adenosine monophosphate response element-binding protein in Tregs [333]. Besides, utilizing the AR antagonists and antibodies to target the adenosinergic pathway in preclinical mice models had favorable anti-tumor immune responses and would further enhance the sensitivity of tumors to ICIs therapies in acquired resistance cases [334,335,336,337]. Furthermore, inhibiting the adenosine/A2AR signaling pathway enhanced the efficacy of CAR T cell transfer. Thus, combining an A2AR inhibitor with CAR T cell therapy has shown therapeutic efficacy in preclinical models [338, 339].
Targeting the immune checkpoints on Tregs
Tregs are also with upregulated expression of immune checkpoints. Targeting the immune checkpoints on Treg cells is proposed to be effective for cancers [340,341,342]. For example, CTLA-4 is highly expressed on Treg cells as a co-inhibitory receptor [343]. However, CD47 expression on Tregs is supposed to limit the anti-CTLA-4-mediated elimination. Simultaneously adjusting the “eat me” and “don't eat me” signals leads to the consumption of Tregs in the TME, which may be an efficient strategy for the treatment of solid tumors. Dual targeting of CTLA-4 and CD47 on Tregs preferentially exhausted ICOShigh immunosuppressive Treg cells in the TME and boosted immunity to solid tumors [344]. Tregs restrained the T cell stimulatory activity of APCs by reducing the expression of CD80/CD86 by trogocytosis depending on CTLA-4. The reduction of CD80/CD86 on APCs exerted double suppressive effects on T cell-mediated immune responses by restraining CD80/CD86 co-stimulation to naïve T cells and improving free PD-L1 available on effector T cells. Combination with the blockade of CTLA-4 and PD-1/PD-L1 synergistically inhibited the Tregs-mediated immune suppression and then enhanced the immune responses [345, 346]. The blockade of CTLA-4 triggered CD28-dependent hyper-proliferation of Tregs in the TME, and the inactivation of accompanied Tregs is required to achieve tumor regression. Tregs self-regulate through a feedback loop that relies on CTLA-4 and CD28, which adjusts their accounts based on the amount of local co-stimulation [347,348,349]. Therefore, the disruption by CTLA-4 blockade may provide cancer patients with therapeutic benefits.
Glucocorticoid-induced tumor-necrosis-factor receptor (TNFR)-related protein (GITR, also known as TNFRSF18) is a potential marker of Treg cells. The co-stimulation of GITR-specific antibodies and other co-stimulatory molecules (CD28 or 4-1BB, also known as CD137, or OX40, also known as CD134) on responder T cells function always dominates the co-stimulatory inhibitory functions of Tregs [350, 351]. The combination of anti-TIM-3 ligand galectin-9 and an agonistic GITR antibody depleting Tregs induced the synergistic anti-tumor activity [352]. MK-4166, an anti-human GITR antibody, decreased the numbers of CD44hiICOShi intra-tumoral Tregs and immune suppressor phenotype while enhancing the effector responsiveness. Intra-tumoral CD8+ T cells acquire a more functional phenotype featured by downregulation of the exhaustion markers PD-1 and LAG-3 [353]. The phase I trial of an anti-GITR antibody, TRX518 (NCT01239134) monotherapy in patients with advanced cancer, demonstrated that TRX518 reduced the circulating and intra-tumoral Tregs. However, the revival of T cells with PD-L1 blockade overwhelms the resistance of advanced cancers to anti-GITR monotherapy. Based on this evidence, the clinical trial using TRX518 and PD-1 blockade in patients with advanced/refractory solid tumors started (NCT02628574) [354]. Treatment with adenovirus-based vaccine plus N-803, OX40, GITR, and IDO inhibitor resulted in decreased immunosuppression of Tregs in the TME, and then the inhibition of tumor growth and protection from cancer cells rechallenge in 4T1 and LL2 models. Monotherapy with each of these agents had limited anti-tumor efficacy. However, the combination of these agents has increased anti-tumor activity, which provides the rationale for the combination of multi-modal immunotherapies to enable adaptive anti-tumor immunity [355].
Tregs express LAG-3, a CD4-associated molecule that binds to MHC-II, contributing to Tregs suppressor activity. LAG-3 antibodies inhibited the suppression by induced Treg cells both in vitro and in vivo [356]. Antibody-mediated elimination of 4-1BB-expressing Tregs decreased the tumor growth without negatively affecting the function of CD8+ T cells representing a strategy with potential anti-tumor activity [357]. When comparing anti-tumor activity between the anti-4-1BB/anti-PD-1 and the anti-PD-1/anti-LAG-3 in the B16-F10 melanoma mouse model, tumor regression occurred in animals receiving anti-PD-1 anti-4-1BB was more concomitantly [358].
TIGIT is also over-expressed on Treg cells and associated with immunosuppression [359, 360]. Anti-TIGIT/CD155 pathway treatment significantly inhibited tumor growth in mouse models of transgenic head and neck squamous cell carcinoma. It improved the anti-tumor immune responses by activating the function of CD8+ T cells and reducing the Tregs [361]. OX40 is expressed with higher levels in murine and human Tregs, promoting Tregs' development and proliferation [362, 363]. Anti-OX40 monoclonal antibody therapies indicated that CD8+ T cell expansion or Tregs consumption might be preferred according to the composition of different cancers [364]. ATOR-1015, a human CTLA-4 and OX40 bi-specific antibody, induced the activation of T cells and depletion of Treg cells, then reduced the tumor growth, and improved the survival in several syngeneic tumor models. ATOR-1015 is expected to have a synergistic effect when combined with anti-PD-1/PD-L1 therapies. Preclinical data suggested that the further clinical application of ATOR-1015 and the first-in-human trial have been launched (NCT03782467) [365]. Combining anti-OX40 and anti-CTLA-4 immunotherapy boosted tumor regression and tumor-bearing host survival depending on CD4+ and CD8+ T cells [366].
Inducible T cell co-stimulator (ICOS) is a highly inhibitory antigen-specific T cell marker with characteristics of Th17/Th1 and Treg cells [367]. ICOS-legend expression by tumor cells directly drove the activation and expansion of Treg in TME [368]. High-dose IL-2 therapy increased the expansion of the ICOS+ Treg population, which may predict clinical outcomes in melanoma [369]. F42K, a mutant IL-2, induced a systematic decrease in the expansion of ICOS+ Treg cells, which promoted the expansion of NK cells and inhibited the tumor growth of melanoma more efficiently than wild-type IL-2 [370]. Akt-mTOR pathway is an essential negative regulator of the differentiation and expansion of Tregs. TCR and IL-2 signals provide the significant inputs for mTORC1 activation, which program the suppressive function of Tregs [371, 372]. KY1044, a fully human IgG1 antibody bounding to ICOS, induced sustained depletion of ICOS+ Treg cells and increased the intra-tumoral Teff/Treg ratio. This KY1044 monotherapy has been demonstrated to improve the anti-PD-L1 efficacy in cancers [373]. Tregs-targeted therapy combined with ICIs can attenuate Treg-mediated drug resistance and increase tumor cell sensitivity to treatment, thereby promoting tumor regression. Several clinical trials are ongoing to investigate the effects of small molecule inhibitors, monoclonal antibodies, multiple ICIs, and combined therapeutics (Table 4).
tDCs-related therapies
DC vaccination
DC vaccines induced an enhanced anti-tumor immune response against tumor antigens. Currently, many clinical trials are designed and ongoing with various DC subsets [374, 375]. For example, a phase I/II study evaluated 10 ovarian cancer patients who received autologous monocyte-derived DC and IL-2 after initial debulking and chemotherapy. Three out of 10 patients showed a sustained complete response, and two patients achieved a partial response. In patients with long-term survival, the improved immunologic characteristics, including increased activity of NK cells, increased IFN-γ-secreting T cells, immunostimulatory cytokine secretion, and decreased secretion of immunosuppressive factors, were observed after DC vaccination [376]. A phase I study (NCT02285413) involved patients with stage III/IV melanoma who received autologous monocyte-derived DC vaccination. The antigen-specific CD8+ T cells were found in 44% versus 67%, T cell responses in 28% versus 19% in patients who received DC vaccination with and without cisplatin. Combination therapy with DC vaccination and cisplatin in melanoma resulted in more tumor-specific T cell responses than the monotherapy of DC vaccination [377]. A phase I study resembling a viral infection in tumor tissue combined with a DC vaccine and ablative radiotherapy showed immune-associated activity and preliminary clinical therapeutic effect signs. Nine out of 15 pre-treated patients with castration-resistant prostate cancer presented stable disease [378]. Gene and protein expression analyses in lymphocytes and cancer samples after DC vaccination identified vital immunoregulatory pathways, including PD-1 and CTLA-4, which correlated with inferior clinical outcomes [379]. In addition, a phase I trial (NCT01946373) investigated the safety/feasibility of TILs adoptive cell therapy combined with DC vaccination in patients with melanoma progressing after ICIs. Two out of 5 patients experienced complete responses. Two patients had partial responses [380]. A preclinical study has shown the promising efficacy of DC vaccination in combination with CD40-stimulation for pancreatic cancer and deserves future clinical trials [381]. Despite the success of DC vaccines in multiple preclinical studies and clinical trials, there are many challenges associated with them, including the lack of a universal vaccine, the high cost of treatment, the need for a large number of DCs, and the difficulty of isolating cDCs.
Reprogramming tDCs to be immunostimulatory with nanoparticles
Isolating and reprogramming DCs to induce tolerogenic DCs are a novel immunotherapy strategy for cancers. Nanomedicines targeting DCs to perform this reprogramming process induced comprehensive immuno-modulations [382]. Lipid nanoparticle delivery of siRNAs with a single-chain antibody to target DCs restricted their uptake and conferred profound gene knockdown to restrain robust immune responses [383]. Currently, modification of adjuvant imiquimod (R837) plus immunomodulator 1-methyl-d-tryptophan (1 MT)@ZIF-8 with mannan to achieve DCs-targeted immune amplification, which adequately activated the immune response and supplied effective platform against advanced malignancies [384]. In addition, nanoparticles display that anti-DC-specific intracellular adhesion molecule-3-grabbing non-integrin (DC-SIGN; CD209) antibody loaded with rapamycin (sirolimus) is favorable to modify DCs. Rapamycin-loaded DC-SIGN nanoparticles' uptake efficiency into DCs resulted in a maturation resistant phenotype and inhibited the extend of allogeneic T cells [385].
In conclusion, reprogrammed tDCs with nanoparticles can elicit enhanced anti-tumor immunity, which may serve as a novel therapeutic strategy to suppress malignant cell survival. Nevertheless, there are still some problems that limit the application of nanoparticles. For example, the persistence of nanoparticle-induced immune tolerance may involve multiple nanoparticle administrations; the long-term impact of nanoparticle safety and side effects needs to be further assessed; there may be difficulties with the manufacture and regulatory approval of complexed nanoparticles.
Conclusions and perspectives
With the development of immunotherapies, the critical roles of immunosuppressive during cancer immunotherapies have been revealed. The efficacy of targeting these immunosuppressive cells to increase the effectiveness of immunotherapies or reverse the resistance of immunotherapies is promising and encouraging. However, the infiltration of these immunosuppressive cells is associated with cancer types, stages, and the adopted therapies. Thus, individually analyzing the TME of advanced tumors, identifying the phenotype of these immunosuppressive cells in TME is critical for developing related treatment. In addition, the generation, expansion, recruitment, and activation of immunosuppressive cells in the tumor microenvironment involve complex mechanisms, making it difficult for a single strategy to target any one of these cells to induce robust anti-tumor effects [386]. Therefore, immunosuppressive cell-targeted therapy combined with other anti-tumor therapies is the preferred strategy. At this time, the dose, timing, and sequence of treatment should be fully considered. Besides, totally deleting these cells may cause universal loss of innate immune for the overlap targets between immunosuppressive cells in TME and the organ resident innate immune cells. The solution must be a complex combination; in addition to supporting the inflammatory phenotype of immune cells, it also includes the selective suppression or consumption of powerful tumor suppressor cytokines and cell types.
Second, there have overlaps of targets among different immunosuppressive cells, like M-MDSCs and M2-TAMs. Thus, some dual targets are proposed to have better efficacy, like CSF-1R. For the engineered CAR-M, which is still at its nascent stage with limited clinical trials initiated, the limitations of CAR-M have yet to be unfolded. For Tregs targeting, the therapies are predominately focused on the ICIs that are expressed on Tregs. tDCs-related treatments mainly focused on DC-related vaccines to modulate the TME. The process of establishing clinically applicable DC vaccines is complex and with high technical requirements. Another issue needed to emphasize is the sequence of administration of these therapies based on immunosuppressive cell, and the efficacy based on these therapies largely depends on the availability of appropriate drug delivery strategies. Besides, suspension of treatment may lead to recovery of the TME and increase disease progression. Overall, targeting immunosuppressive cells has shown synergistic anti-tumor efficacy with immunotherapies. However, the further mechanisms of these cells participating in the tumor immune response and the optimization of clinical application of therapies targeting these cells are needed.
Availability of data and materials
The materials supporting our conclusion of this review are included within the article.
Abbreviations
- MDSCs:
-
Myeloid-derived suppressive cells
- TAMs:
-
Tumor-associated macrophages
- TANs:
-
Tumor-associated neutrophils
- Tregs:
-
Regulatory T cells
- tDCs:
-
Tumor-associated dendritic cells
- ICIs:
-
Immune checkpoint inhibitors
- TME:
-
Tumor microenvironment
- VEGF:
-
Vascular endothelial growth factor
- TGF-β:
-
Transforming growth factor beta
- IL-10:
-
Interleukin-10
- EMT:
-
Epithelial–mesenchymal transition
- MMPs:
-
Matrix metalloproteinases
- NETs:
-
Neutrophil extracellular traps
- PGE2:
-
Prostaglandin E2
- TIM-3:
-
T cell immunoglobulin and mucin domain 3
- 5-FU:
-
5-Fluorouracil
- BTK:
-
Bruton's tyrosine kinase
- NLRP3:
-
Nucleotide oligomerization domain-like receptor family pyrin domain-containing 3
- PDE5:
-
Phosphodiesterase-5
- HDAC:
-
Histone deacetylase inhibitor
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- ATRA:
-
All-trans-retinoic acid
- CK2:
-
Casein kinase 2
- LXR:
-
Liver-X nuclear receptor
- CTL:
-
Cytotoxic T lymphocyte
- ApoE:
-
Apolipoprotein E
- LRP:
-
Lipoprotein receptor-related protein
- FATP2:
-
Fatty acid transport protein 2
- IDO:
-
Indoleamine 2,3-dioxygenase
- SIRPα:
-
Signal regulatory protein alpha
- Siglec-10:
-
Sialic-acid-binding Ig-like lectin 10
- ADCP:
-
Antibody-dependent cellular phagocytosis
- CAR:
-
Chimeric antigen receptor
- FRβ:
-
Folate receptor β
- ADCC:
-
Antibody dependent cellular cytotoxicity
- VISTA:
-
V-domain immunoglobulin suppressor of T cell activation
- TCR:
-
T cell antigen receptor
- TTP:
-
Time to progression
- ORR:
-
Objective response rate
- TILs:
-
Tumor-infiltrating lymphocytes
- LAG-3:
-
Lymphocyte activation gene 3
- TIGIT:
-
T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain
- TNFR:
-
Tumor-necrosis-factor receptor
- GITR:
-
Glucocorticoid-induced tumor-necrosis-factor receptor;
- ICOS:
-
Inducible T cell co-stimulator
References
Heeren AM, Rotman J, Samuels S, et al. Immune landscape in vulvar cancer-draining lymph nodes indicates distinct immune escape mechanisms in support of metastatic spread and growth. J Immunother Cancer. 2021;9(10):e003623.
Tumino N, Weber G, Besi F, et al. Polymorphonuclear myeloid-derived suppressor cells impair the anti-tumor efficacy of GD2.CAR T-cells in patients with neuroblastoma. J Hematol Oncol. 2021;14(1):191.
Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–8.
Valencia JC, Erwin-Cohen RA, Clavijo PE, et al. Myeloid derived suppressive cell expansion promotes melanoma growth and severity of autoimmunity by inhibiting CD40/IL-27 regulation in macrophages. Cancer Res. 2021;81(23):5977–90.
Li S, Liu M, Do MH, et al. Cancer immunotherapy via targeted TGF-β signalling blockade in T(H) cells. Nature. 2020;587(7832):121–5.
Aguilera KY, Rivera LB, Hur H, et al. Collagen signaling enhances tumor progression after anti-VEGF therapy in a murine model of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74(4):1032–44.
Ouyang W, O’Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity. 2019;50(4):871–91.
Sade-Feldman M, Jiao YJ, Chen JH, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun. 2017;8(1):1136.
Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35(Suppl):S185–98.
Youn JI, Collazo M, Shalova IN, et al. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91(1):167–81.
Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150.
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–68.
Kumar V, Patel S, Tcyganov E, et al. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208–20.
Vetsika EK, Koukos A, Kotsakis A. Myeloid-derived suppressor cells: major figures that shape the immunosuppressive and angiogenic network in cancer. Cells. 2019;8(12):1647.
Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19(2):108–19.
Nakamura K, Smyth MJ. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol. 2020;17(1):1–12.
Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79(18):4557–66.
Taki M, Abiko K, Ukita M, et al. Tumor immune microenvironment during epithelial–mesenchymal transition. Clin Cancer Res. 2021;27(17):4669–79.
Tang F, Tie Y, Hong W, et al. Targeting myeloid-derived suppressor cells for premetastatic niche disruption after tumor resection. Ann Surg Oncol. 2021;28(7):4030–48.
Güç E, Pollard JW. Redefining macrophage and neutrophil biology in the metastatic cascade. Immunity. 2021;54(5):885–902.
Jiménez-Cortegana C, Palazón-Carrión N, Martin Garcia-Sancho A, et al. Circulating myeloid-derived suppressor cells and regulatory T cells as immunological biomarkers in refractory/relapsed diffuse large B-cell lymphoma: translational results from the R2-GDP-GOTEL trial. J Immunother Cancer. 2021;9(6).
Kim W, Chu TH, Nienhüser H, et al. PD-1 signaling promotes tumor-infiltrating myeloid-derived suppressor cells and gastric tumorigenesis in mice. Gastroenterology. 2021;160(3):781–96.
Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5(1):3–8.
Apodaca MC, Wright AE, Riggins AM, et al. Characterization of a whole blood assay for quantifying myeloid-derived suppressor cells. J Immunother Cancer. 2019;7(1):230.
Bae MH, Park CJ, Suh C. Increased monocytic myeloid-derived suppressor cells in whole blood predict poor prognosis in patients with plasma cell myeloma. J Clin Med. 2021;10(20).
Chen IX, Newcomer K, Pauken KE, et al. A bilateral tumor model identifies transcriptional programs associated with patient response to immune checkpoint blockade. Proc Natl Acad Sci USA. 2020;117(38):23684–94.
Peranzoni E, Ingangi V, Masetto E, et al. Myeloid cells as clinical biomarkers for immune checkpoint blockade. Front Immunol. 2020;11:1590.
Cheng N, Bai X, Shu Y, et al. Targeting tumor-associated macrophages as an antitumor strategy. Biochem Pharmacol. 2021;183: 114354.
Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17(12):887–904.
Cardoso AP, Pinto ML, Pinto AT, et al. Macrophages stimulate gastric and colorectal cancer invasion through EGFR Y(1086), c-Src, Erk1/2 and Akt phosphorylation and smallGTPase activity. Oncogene. 2014;33(16):2123–33.
Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10(1):58.
Kim HY, Lee HJ, Chang YJ, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20(1):54–61.
Funes SC, Rios M, Escobar-Vera J, et al. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154(2):186–95.
Hartley GP, Chow L, Ammons DT, et al. Programmed cell death ligand 1 (PD-L1) signaling regulates macrophage proliferation and activation. Cancer Immunol Res. 2018;6(10):1260–73.
Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017;114:206–21.
Loeuillard E, Yang J, Buckarma E, et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J Clin Invest. 2020;130(10):5380–96.
Jaillon S, Ponzetta A, Di Mitri D, et al. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20(9):485–503.
Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019;16(10):601–20.
Lee W, Ko SY, Mohamed MS, et al. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. 2019;216(1):176–94.
Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33(5):949–55.
Keeley T, Costanzo-Garvey DL, Cook LM. Unmasking the many faces of tumor-associated neutrophils and macrophages: considerations for targeting innate immune cells in cancer. Trends Cancer. 2019;5(12):789–98.
Rogers T, DeBerardinis RJ. Metabolic plasticity of neutrophils: relevance to pathogen responses and cancer. Trends Cancer. 2021;7(8):700–13.
Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–94.
Finisguerra V, Di Conza G, Di Matteo M, et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature. 2015;522(7556):349–53.
Tecchio C, Scapini P, Pizzolo G, et al. On the cytokines produced by human neutrophils in tumors. Semin Cancer Biol. 2013;23(3):159–70.
Albrengues J, Shields MA, Ng D, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409):eaao4227.
Schauer C, Janko C, Munoz LE, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20(5):511–7.
Demers M, Wong SL, Martinod K, et al. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology. 2016;5(5): e1134073.
Ma X, Aoki T, Tsuruyama T, et al. Definition of prostaglandin E2-EP2 signals in the colon tumor microenvironment that amplify inflammation and tumor growth. Cancer Res. 2015;75(14):2822–32.
Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA. 2006;103(33):12493–8.
Queen MM, Ryan RE, Holzer RG, et al. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65(19):8896–904.
Sharabi A, Tsokos MG, Ding Y, et al. Regulatory T cells in the treatment of disease. Nat Rev Drug Discov. 2018;17(11):823–44.
Li C, Jiang P, Wei S, et al. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020;19(1):116.
Raffin C, Vo LT, Bluestone JA. T(reg) cell-based therapies: challenges and perspectives. Nat Rev Immunol. 2020;20(3):158–72.
Wing JB, Tanaka A, Sakaguchi S. Human FOXP3(+) regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50(2):302–16.
Kim JH, Hwang J, Jung JH, et al. Molecular networks of FOXP family: dual biologic functions, interplay with other molecules and clinical implications in cancer progression. Mol Cancer. 2019;18(1):180.
Grinberg-Bleyer Y, Oh H, Desrichard A, et al. NF-κB c-Rel Is crucial for the regulatory T cell immune checkpoint in cancer. Cell. 2017;170(6):1096-108.e13.
Noval Rivas M, Burton OT, Wise P, et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015;42(3):512–23.
Beriou G, Costantino CM, Ashley CW, et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113(18):4240–9.
Di Pilato M, Kim EY, Cadilha BL, et al. Targeting the CBM complex causes T(reg) cells to prime tumours for immune checkpoint therapy. Nature. 2019;570(7759):112–6.
Saleh R, Elkord E. Treg-mediated acquired resistance to immune checkpoint inhibitors. Cancer Lett. 2019;457:168–79.
Wang H, Franco F, Ho PC. Metabolic regulation of Tregs in cancer: opportunities for immunotherapy. Trends Cancer. 2017;3(8):583–92.
Toomer KH, Malek TR. Cytokine signaling in the development and homeostasis of regulatory T cells. Cold Spring Harb Perspect Biol. 2018;10(3):a028597.
Budhu S, Schaer DA, Li Y, et al. Blockade of surface-bound TGF-β on regulatory T cells abrogates suppression of effector T cell function in the tumor microenvironment. Sci Signal. 2017;10(494):eaak9702.
Knochelmann HM, Dwyer CJ, Bailey SR, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol. 2018;15(5):458–69.
Kalia V, Penny LA, Yuzefpolskiy Y, et al. Quiescence of memory CD8(+) T cells is mediated by regulatory T cells through inhibitory receptor CTLA-4. Immunity. 2015;42(6):1116–29.
Spolski R, Li P, Leonard WJ. Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nat Rev Immunol. 2018;18(10):648–59.
Conejo-Garcia JR, Rutkowski MR, Cubillos-Ruiz JR. State-of-the-art of regulatory dendritic cells in cancer. Pharmacol Ther. 2016;164:97–104.
Keirsse J, Van Damme H, Van Ginderachter JA, et al. Exploiting tumor-associated dendritic cell heterogeneity for novel cancer therapies. J Leukoc Biol. 2017;102(2):317–24.
Laoui D, Keirsse J, Morias Y, et al. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat Commun. 2016;7:13720.
Scarlett UK, Rutkowski MR, Rauwerdink AM, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209(3):495–506.
Saleh R, Elkord E. Acquired resistance to cancer immunotherapy: role of tumor-mediated immunosuppression. Semin Cancer Biol. 2020;65:13–27.
Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4(12):941–52.
Sumpter TL, Dangi A, Matta BM, et al. Hepatic stellate cells undermine the allostimulatory function of liver myeloid dendritic cells via STAT3-dependent induction of IDO. J Immunol. 2012;189(8):3848–58.
Pedroza-Gonzalez A, Zhou G, Vargas-Mendez E, et al. Tumor-infiltrating plasmacytoid dendritic cells promote immunosuppression by Tr1 cells in human liver tumors. Oncoimmunology. 2015;4(6): e1008355.
Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018;154(1):3–20.
O’Keeffe M, Mok WH, Radford KJ. Human dendritic cell subsets and function in health and disease. Cell Mol Life Sci. 2015;72(22):4309–25.
Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607–12.
Shang J, Zha H, Sun Y. Phenotypes, functions, and clinical relevance of regulatory B cells in cancer. Front Immunol. 2020;11:582657.
Khan AR, Hams E, Floudas A, et al. PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun. 2015;6:5997.
Carter NA, Rosser EC, Mauri C. Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Res Ther. 2012;14(1):R32.
Pittoni P, Colombo MP. The dark side of mast cell-targeted therapy in prostate cancer. Cancer Res. 2012;72(4):831–5.
Oldford SA, Marshall JS. Mast cells as targets for immunotherapy of solid tumors. Mol Immunol. 2015;63(1):113–24.
Li K, Shi H, Zhang B, et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6(1):362.
Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052–61.
Eriksson E, Wenthe J, Irenaeus S, et al. Gemcitabine reduces MDSCs, tregs and TGFβ-1 while restoring the teff/treg ratio in patients with pancreatic cancer. J Transl Med. 2016;14(1):282.
Sevko A, Michels T, Vrohlings M, et al. Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J Immunol. 2013;190(5):2464–71.
Sasso MS, Lollo G, Pitorre M, et al. Low dose gemcitabine-loaded lipid nanocapsules target monocytic myeloid-derived suppressor cells and potentiate cancer immunotherapy. Biomaterials. 2016;96:47–62.
Ko JS, Rayman P, Ireland J, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70(9):3526–36.
Stiff A, Trikha P, Wesolowski R, et al. Myeloid-derived suppressor cells express bruton’s tyrosine kinase and can be depleted in tumor-bearing hosts by ibrutinib treatment. Cancer Res. 2016;76(8):2125–36.
Rinchai D, Verzoni E, Huber V, et al. Integrated transcriptional-phenotypic analysis captures systemic immunomodulation following antiangiogenic therapy in renal cell carcinoma patients. Clin Transl Med. 2021;11(6): e434.
Dominguez GA, Condamine T, Mony S, et al. Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody. Clin Cancer Res. 2017;23(12):2942–50.
Hartwig T, Montinaro A, von Karstedt S, et al. The TRAIL-induced cancer secretome promotes a tumor-supportive immune microenvironment via CCR2. Mol Cell. 2017;65(4):730-42.e5.
Eksioglu EA, Chen X, Heider KH, et al. Novel therapeutic approach to improve hematopoiesis in low risk MDS by targeting MDSCs with the Fc-engineered CD33 antibody BI 836858. Leukemia. 2017;31(10):2172–80.
Fultang L, Panetti S, Ng M, et al. MDSC targeting with Gemtuzumab ozogamicin restores T cell immunity and immunotherapy against cancers. EBioMedicine. 2019;47:235–46.
Qin H, Lerman B, Sakamaki I, et al. Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med. 2014;20(6):676–81.
Katoh H, Wang D, Daikoku T, et al. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24(5):631–44.
Steele CW, Karim SA, Leach JDG, et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell. 2016;29(6):832–45.
Sun L, Clavijo PE, Robbins Y, et al. Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight. 2019;4(7):e126853.
Greene S, Robbins Y, Mydlarz WK, et al. Inhibition of MDSC trafficking with SX-682, a CXCR1/2 inhibitor, enhances nk-cell immunotherapy in head and neck cancer models. Clin Cancer Res. 2020;26(6):1420–31.
Teijeira Á, Garasa S, Gato M, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. 2020;52(5):856-71.e8.
Schott AF, Goldstein LJ, Cristofanilli M, et al. Phase Ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with HER-2-negative metastatic breast cancer. Clin Cancer Res. 2017;23(18):5358–65.
Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME, et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev. 2017;60:24–31.
Bilusic M, Heery CR, Collins JM, et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J Immunother Cancer. 2019;7(1):240.
Blattner C, Fleming V, Weber R, et al. CCR5(+) myeloid-derived suppressor cells are enriched and activated in melanoma lesions. Cancer Res. 2018;78(1):157–67.
Ban Y, Mai J, Li X, et al. Targeting autocrine CCL5-CCR5 axis reprograms immunosuppressive myeloid cells and reinvigorates antitumor immunity. Cancer Res. 2017;77(11):2857–68.
Velasco-Velázquez M, Jiao X, De La Fuente M, et al. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res. 2012;72(15):3839–50.
Flores-Toro JA, Luo D, Gopinath A, et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc Natl Acad Sci USA. 2020;117(2):1129–38.
Holmgaard RB, Zamarin D, Lesokhin A, et al. Targeting myeloid-derived suppressor cells with colony stimulating factor-1 receptor blockade can reverse immune resistance to immunotherapy in indoleamine 2,3-dioxygenase-expressing tumors. EBioMedicine. 2016;6:50–8.
Kumar V, Donthireddy L, Marvel D, et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell. 2017;32(5):654-68.e5.
Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74(18):5057–69.
Priceman SJ, Sung JL, Shaposhnik Z, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115(7):1461–71.
Horikawa N, Abiko K, Matsumura N, et al. Expression of vascular endothelial growth factor in ovarian cancer inhibits tumor immunity through the accumulation of myeloid-derived suppressor cells. Clin Cancer Res. 2017;23(2):587–99.
Rivera LB, Bergers G. Intertwined regulation of angiogenesis and immunity by myeloid cells. Trends Immunol. 2015;36(4):240–9.
Limagne E, Euvrard R, Thibaudin M, et al. Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-bevacizumab drug treatment regimen. Cancer Res. 2016;76(18):5241–52.
Koinis F, Vetsika EK, Aggouraki D, et al. Effect of first-line treatment on myeloid-derived suppressor cells’ subpopulations in the peripheral blood of patients with non-small cell lung cancer. J Thorac Oncol. 2016;11(8):1263–72.
Peereboom DM, Alban TJ, Grabowski MM, et al. Metronomic capecitabine as an immune modulator in glioblastoma patients reduces myeloid-derived suppressor cells. JCI Insight. 2019;4(22):e130748.
Jiang H, Gebhardt C, Umansky L, et al. Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer. 2015;136(10):2352–60.
Tannenbaum CS, Rayman PA, Pavicic PG, et al. Mediators of inflammation-driven expansion, trafficking, and function of tumor-infiltrating MDSCs. Cancer Immunol Res. 2019;7(10):1687–99.
Shi H, Zhang J, Han X, et al. Recruited monocytic myeloid-derived suppressor cells promote the arrest of tumor cells in the premetastatic niche through an IL-1β-mediated increase in E-selectin expression. Int J Cancer. 2017;140(6):1370–83.
Sota J, Vitale A, Insalaco A, et al. Safety profile of the interleukin-1 inhibitors anakinra and canakinumab in real-life clinical practice: a nationwide multicenter retrospective observational study. Clin Rheumatol. 2018;37(8):2233–40.
Mangan MSJ, Olhava EJ, Roush WR, et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17(8):588–606.
Sinha P, Okoro C, Foell D, et al. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181(7):4666–75.
Bresnick AR, Weber DJ, Zimmer DB. S100 proteins in cancer. Nat Rev Cancer. 2015;15(2):96–109.
Kinoshita R, Sato H, Yamauchi A, et al. Newly developed anti-S100A8/A9 monoclonal antibody efficiently prevents lung tropic cancer metastasis. Int J Cancer. 2019;145(2):569–75.
Shen L, Pili R. Tasquinimod targets suppressive myeloid cells in the tumor microenvironment. Oncoimmunology. 2019;8(10): e1072672.
Escudier B, Faivre S, Van Cutsem E, et al. A phase II multicentre, open-label, proof-of-concept study of tasquinimod in hepatocellular, ovarian, renal cell, and gastric cancers. Target Oncol. 2017;12(5):655–61.
Pili R, Häggman M, Stadler WM, et al. Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. J Clin Oncol. 2011;29(30):4022–8.
Sternberg C, Armstrong A, Pili R, et al. Randomized, double-blind, placebo-controlled phase III study of tasquinimod in men with metastatic castration-resistant prostate cancer. J Clin Oncol. 2016;34(22):2636–43.
Reilley MJ, McCoon P, Cook C, et al. STAT3 antisense oligonucleotide AZD9150 in a subset of patients with heavily pretreated lymphoma: results of a phase 1b trial. J Immunother Cancer. 2018;6(1):119.
Hossain DM, Pal SK, Moreira D, et al. TLR9-targeted STAT3 silencing abrogates immunosuppressive activity of myeloid-derived suppressor cells from prostate cancer patients. Clin Cancer Res. 2015;21(16):3771–82.
Mace TA, Ameen Z, Collins A, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013;73(10):3007–18.
Guha P, Gardell J, Darpolor J, et al. STAT3 inhibition induces Bax-dependent apoptosis in liver tumor myeloid-derived suppressor cells. Oncogene. 2019;38(4):533–48.
Smith AD, Lu C, Payne D, et al. Autocrine IL6-mediated activation of the STAT3-DNMT axis silences the TNFα-RIP1 necroptosis pathway to sustain survival and accumulation of myeloid-derived suppressor cells. Cancer Res. 2020;80(15):3145–56.
Mohrherr J, Haber M, Breitenecker K, et al. JAK-STAT inhibition impairs K-RAS-driven lung adenocarcinoma progression. Int J Cancer. 2019;145(12):3376–88.
Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203(12):2691–702.
Weed DT, Vella JL, Reis IM, et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21(1):39–48.
Obermajer N, Muthuswamy R, Lesnock J, et al. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118(20):5498–505.
Fujita M, Kohanbash G, Fellows-Mayle W, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71(7):2664–74.
Wong JL, Obermajer N, Odunsi K, et al. Synergistic COX2 induction by IFNγ and TNFα self-limits type-1 immunity in the human tumor microenvironment. Cancer Immunol Res. 2016;4(4):303–11.
Prima V, Kaliberova LN, Kaliberov S, et al. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci U S A. 2017;114(5):1117–22.
Youn JI, Kumar V, Collazo M, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. 2013;14(3):211–20.
Lu Z, Zou J, Li S, et al. Epigenetic therapy inhibits metastases by disrupting premetastatic niches. Nature. 2020;579(7798):284–90.
Li X, Su X, Liu R, et al. HDAC inhibition potentiates anti-tumor activity of macrophages and enhances anti-PD-L1-mediated tumor suppression. Oncogene. 2021;40(10):1836–50.
Orillion A, Hashimoto A, Damayanti N, et al. Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin Cancer Res. 2017;23(17):5187–201.
Christmas BJ, Rafie CI, Hopkins AC, et al. Entinostat converts immune-resistant breast and pancreatic cancers into checkpoint-responsive tumors by reprogramming tumor-infiltrating MDSCs. Cancer Immunol Res. 2018;6(12):1561–77.
Hiramoto K, Satoh H, Suzuki T, et al. Myeloid lineage-specific deletion of antioxidant system enhances tumor metastasis. Cancer Prev Res (Phila). 2014;7(8):835–44.
Beury DW, Carter KA, Nelson C, et al. Myeloid-derived suppressor cell survival and function are regulated by the transcription factor Nrf2. J Immunol. 2016;196(8):3470–8.
Nagaraj S, Youn JI, Weber H, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16(6):1812–23.
Diaz-Montero CM, Wang Y, Shao L, et al. The glutathione disulfide mimetic NOV-002 inhibits cyclophosphamide-induced hematopoietic and immune suppression by reducing oxidative stress. Free Radic Biol Med. 2012;52(9):1560–8.
Montero AJ, Diaz-Montero CM, Deutsch YE, et al. Phase 2 study of neoadjuvant treatment with NOV-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with HER-2 negative clinical stage II-IIIc breast cancer. Breast Cancer Res Treat. 2012;132(1):215–23.
Nefedova Y, Fishman M, Sherman S, et al. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 2007;67(22):11021–8.
Bauer R, Udonta F, Wroblewski M, et al. Blockade of myeloid-derived suppressor cell expansion with all-trans retinoic acid increases the efficacy of antiangiogenic therapy. Cancer Res. 2018;78(12):3220–32.
Tobin RP, Jordan KR, Robinson WA, et al. Targeting myeloid-derived suppressor cells using all-trans retinoic acid in melanoma patients treated with Ipilimumab. Int Immunopharmacol. 2018;63:282–91.
Long AH, Highfill SL, Cui Y, et al. Reduction of MDSCs with all-trans retinoic acid improves CAR therapy efficacy for sarcomas. Cancer Immunol Res. 2016;4(10):869–80.
Li R, Salehi-Rad R, Crosson W, et al. Inhibition of granulocytic myeloid-derived suppressor cells overcomes resistance to immune checkpoint inhibition in LKB1-deficient non-small cell lung cancer. Cancer Res. 2021;81(12):3295–308.
Cheng P, Kumar V, Liu H, et al. Effects of notch signaling on regulation of myeloid cell differentiation in cancer. Cancer Res. 2014;74(1):141–52.
Hashimoto A, Gao C, Mastio J, et al. Inhibition of casein kinase 2 disrupts differentiation of myeloid cells in cancer and enhances the efficacy of immunotherapy in mice. Cancer Res. 2018;78(19):5644–55.
Shayan G, Kansy BA, Gibson SP, et al. Phase Ib study of immune biomarker modulation with neoadjuvant cetuximab and TLR8 stimulation in head and neck cancer to overcome suppressive myeloid signals. Clin Cancer Res. 2018;24(1):62–72.
Lee M, Park CS, Lee YR, et al. Resiquimod, a TLR7/8 agonist, promotes differentiation of myeloid-derived suppressor cells into macrophages and dendritic cells. Arch Pharm Res. 2014;37(9):1234–40.
Di S, Zhou M, Pan Z, et al. Combined adjuvant of poly I: C improves antitumor effects of CAR-T cells. Front Oncol. 2019;9:241.
Domankevich V, Cohen A, Efrati M, et al. Combining alpha radiation-based brachytherapy with immunomodulators promotes complete tumor regression in mice via tumor-specific long-term immune response. Cancer Immunol Immunother. 2019;68(12):1949–58.
Forghani P, Waller EK. Poly (I: C) modulates the immunosuppressive activity of myeloid-derived suppressor cells in a murine model of breast cancer. Breast Cancer Res Treat. 2015;153(1):21–30.
Gao W, Zhang X, Yang W, et al. Prim-O-glucosylcimifugin enhances the antitumour effect of PD-1 inhibition by targeting myeloid-derived suppressor cells. J Immunother Cancer. 2019;7(1):231.
Tu SP, Jin H, Shi JD, et al. Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev Res (Phila). 2012;5(2):205–15.
Rui K, Tian J, Tang X, et al. Curdlan blocks the immune suppression by myeloid-derived suppressor cells and reduces tumor burden. Immunol Res. 2016;64(4):931–9.
Zhou J, Wu J, Chen X, et al. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int Immunopharmacol. 2011;11(7):890–8.
Albeituni SH, Ding C, Liu M, et al. Yeast-derived particulate β-glucan treatment subverts the suppression of myeloid-derived suppressor cells (MDSC) by inducing polymorphonuclear mdsc apoptosis and monocytic MDSC differentiation to APC in cancer. J Immunol. 2016;196(5):2167–80.
Tavazoie MF, Pollack I, Tanqueco R, et al. LXR/ApoE activation restricts innate immune suppression in cancer. Cell. 2018;172(4):825-40.e18.
LXR agonism depletes MDSCs to promote antitumor immunity. Cancer Discov. 2018;8(3):263.
Hossain F, Al-Khami AA, Wyczechowska D, et al. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol Res. 2015;3(11):1236–47.
Veglia F, Tyurin VA, Blasi M, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature. 2019;569(7754):73–8.
Al-Khami AA, Rodriguez PC, Ochoa AC. Metabolic reprogramming of myeloid-derived suppressor cells (MDSC) in cancer. Oncoimmunology. 2016;5(8): e1200771.
Salminen A, Kauppinen A, Kaarniranta K. AMPK activation inhibits the functions of myeloid-derived suppressor cells (MDSC): impact on cancer and aging. J Mol Med (Berl). 2019;97(8):1049–64.
Jian SL, Chen WW, Su YC, et al. Glycolysis regulates the expansion of myeloid-derived suppressor cells in tumor-bearing hosts through prevention of ROS-mediated apoptosis. Cell Death Dis. 2017;8(5): e2779.
Qin G, Lian J, Huang L, et al. Metformin blocks myeloid-derived suppressor cell accumulation through AMPK-DACH1-CXCL1 axis. Oncoimmunology. 2018;7(7):e1442167.
Xu P, Yin K, Tang X, et al. Metformin inhibits the function of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Biomed Pharmacother. 2019;120: 109458.
Théate I, van Baren N, Pilotte L, et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol Res. 2015;3(2):161–72.
Holmgaard RB, Zamarin D, Li Y, et al. Tumor-expressed IDO recruits and activates MDSCs in a Treg-dependent manner. Cell Rep. 2015;13(2):412–24.
Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37(3):193–207.
Li F, Zhao Y, Wei L, et al. Tumor-infiltrating Treg, MDSC, and IDO expression associated with outcomes of neoadjuvant chemotherapy of breast cancer. Cancer Biol Ther. 2018;19(8):695–705.
Labadie BW, Bao R, Luke JJ. Reimagining IDO pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan–kynurenine–aryl hydrocarbon axis. Clin Cancer Res. 2019;25(5):1462–71.
Mariotti V, Han H, Ismail-Khan R, et al. Effect of taxane chemotherapy with or without indoximod in metastatic breast cancer: a randomized clinical trial. JAMA Oncol. 2021;7(1):61–9.
Ricciuti B, Leonardi GC, Puccetti P, et al. Targeting indoleamine-2,3-dioxygenase in cancer: scientific rationale and clinical evidence. Pharmacol Ther. 2019;196:105–16.
Cheong JE, Sun L. Targeting the IDO1/TDO2-KYN-AhR pathway for cancer immunotherapy—challenges and opportunities. Trends Pharmacol Sci. 2018;39(3):307–25.
Le Naour J, Galluzzi L, Zitvogel L, et al. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2020;9(1):1777625.
Qian Y, Qiao S, Dai Y, et al. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano. 2017;11(9):9536–49.
Peng X, Hou P, Chen Y, et al. Preclinical evaluation of 3D185, a novel potent inhibitor of FGFR1/2/3 and CSF-1R, in FGFR-dependent and macrophage–dominant cancer models. J Exp Clin Cancer Res. 2019;38(1):372.
Dammeijer F, Lievense LA, Kaijen-Lambers ME, et al. Depletion of tumor-associated macrophages with a CSF-1R kinase inhibitor enhances antitumor immunity and survival induced by DC immunotherapy. Cancer Immunol Res. 2017;5(7):535–46.
Gomez-Roca CA, Italiano A, Le Tourneau C, et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann Oncol. 2019;30(8):1381–92.
Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–72.
Akkari L, Bowman RL, Tessier J, et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci Transl Med. 2020;2(552):eaaw7843.
Li F, Kitajima S, Kohno S, et al. Retinoblastoma inactivation induces a protumoral microenvironment via enhanced CCL2 secretion. Cancer Res. 2019;79(15):3903–15.
Schmall A, Al-Tamari HM, Herold S, et al. Macrophage and cancer cell cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving lung cancer. Am J Respir Crit Care Med. 2015;191(4):437–47.
Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–72.
Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73(3):1128–41.
Li X, Yao W, Yuan Y, et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66(1):157–67.
Yao W, Ba Q, Li X, et al. A natural CCR2 antagonist relieves tumor-associated macrophage-mediated immunosuppression to produce a therapeutic effect for liver cancer. EBioMedicine. 2017;22:58–67.
Nywening TM, Belt BA, Cullinan DR, et al. Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut. 2018;67(6):1112–23.
Chen Y, Huang Y, Reiberger T, et al. Differential effects of sorafenib on liver versus tumor fibrosis mediated by stromal-derived factor 1 alpha/C-X-C receptor type 4 axis and myeloid differentiation antigen-positive myeloid cell infiltration in mice. Hepatology. 2014;59(4):1435–47.
Xiang X, Wang J, Lu D, et al. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. 2021;6(1):75.
Chen Y, Ramjiawan RR, Reiberger T, et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology. 2015;61(5):1591–602.
Meng YM, Liang J, Wu C, et al. Monocytes/macrophages promote vascular CXCR4 expression via the ERK pathway in hepatocellular carcinoma. Oncoimmunology. 2018;7(3):e1408745.
Tian L, Yi X, Dong Z, et al. Calcium bisphosphonate nanoparticles with chelator-free radiolabeling to deplete tumor-associated macrophages for enhanced cancer radioisotope therapy. ACS Nano. 2018;12(11):11541–51.
Junankar S, Shay G, Jurczyluk J, et al. Real-time intravital imaging establishes tumor-associated macrophages as the extraskeletal target of bisphosphonate action in cancer. Cancer Discov. 2015;5(1):35–42.
Lv J, Chen FK, Liu C, et al. Zoledronic acid inhibits thyroid cancer stemness and metastasis by repressing M2-like tumor-associated macrophages induced Wnt/β-catenin pathway. Life Sci. 2020;256: 117925.
Marra M, Salzano G, Leonetti C, et al. New self-assembly nanoparticles and stealth liposomes for the delivery of zoledronic acid: a comparative study. Biotechnol Adv. 2012;30(1):302–9.
Zhan X, Jia L, Niu Y, et al. Targeted depletion of tumour-associated macrophages by an alendronate–glucomannan conjugate for cancer immunotherapy. Biomaterials. 2014;35(38):10046–57.
Allavena P, Signorelli M, Chieppa M, et al. Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005;65(7):2964–71.
Hollander L, Guo X, Velazquez H, et al. Renalase expression by melanoma and tumor-associated macrophages promotes tumor growth through a STAT3-mediated mechanism. Cancer Res. 2016;76(13):3884–94.
Wettersten HI, Weis SM, Pathria P, et al. Arming tumor-associated macrophages to reverse epithelial cancer progression. Cancer Res. 2019;79(19):5048–59.
Baer C, Squadrito ML, Laoui D, et al. Suppression of microRNA activity amplifies IFN-γ-induced macrophage activation and promotes anti-tumour immunity. Nat Cell Biol. 2016;18(7):790–802.
Richman LP, Vonderheide RH. Role of crosslinking for agonistic CD40 monoclonal antibodies as immune therapy of cancer. Cancer Immunol Res. 2014;2(1):19–26.
Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–6.
Perry CJ, Muñoz-Rojas AR, Meeth KM, et al. Myeloid-targeted immunotherapies act in synergy to induce inflammation and antitumor immunity. J Exp Med. 2018;215(3):877–93.
Stromnes IM, Burrack AL, Hulbert A, et al. Differential effects of depleting versus programming tumor-associated macrophages on engineered T cells in pancreatic ductal adenocarcinoma. Cancer Immunol Res. 2019;7(6):977–89.
Ubil E, Caskey L, Holtzhausen A, et al. Tumor-secreted Pros1 inhibits macrophage M1 polarization to reduce antitumor immune response. J Clin Invest. 2018;128(6):2356–69.
Rodell CB, Ahmed MS, Garris CS, et al. Development of adamantane-conjugated TLR7/8 agonists for supramolecular delivery and cancer immunotherapy. Theranostics. 2019;9(26):8426–36.
Muraoka D, Seo N, Hayashi T, et al. Antigen delivery targeted to tumor-associated macrophages overcomes tumor immune resistance. J Clin Invest. 2019;129(3):1278–94.
Chavez M, Silvestrini MT, Ingham ES, et al. Distinct immune signatures in directly treated and distant tumors result from TLR adjuvants and focal ablation. Theranostics. 2018;8(13):3611–28.
Khan MW, Keshavarzian A, Gounaris E, et al. PI3K/AKT signaling is essential for communication between tissue-infiltrating mast cells, macrophages, and epithelial cells in colitis-induced cancer. Clin Cancer Res. 2013;19(9):2342–54.
Ruicci KM, Meens J, Plantinga P, et al. TAM family receptors in conjunction with MAPK signalling are involved in acquired resistance to PI3Kα inhibition in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2020;39(1):217.
Elkabets M, Pazarentzos E, Juric D, et al. AXL mediates resistance to PI3Kα inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell. 2015;27(4):533–46.
Levin PA, Brekken RA, Byers LA, et al. Axl receptor axis: a new therapeutic target in lung cancer. J Thorac Oncol. 2016;11(8):1357–62.
Shi R, Li M, Raghavan V, et al. Targeting the CDK4/6-Rb pathway enhances response to PI3K inhibition in PIK3CA-mutant lung squamous cell carcinoma. Clin Cancer Res. 2018;24(23):5990–6000.
Wang X, Luo X, Chen C, et al. The Ap-2α/Elk-1 axis regulates Sirpα-dependent tumor phagocytosis by tumor-associated macrophages in colorectal cancer. Signal Transduct Target Ther. 2020;5(1):35.
Hutter G, Theruvath J, Graef CM, et al. Microglia are effector cells of CD47-SIRPα antiphagocytic axis disruption against glioblastoma. Proc Natl Acad Sci USA. 2019;116(3):997–1006.
Ma D, Liu S, Lal B, et al. Extracellular matrix protein tenascin C increases phagocytosis mediated by CD47 loss of function in glioblastoma. Cancer Res. 2019;79(10):2697–708.
Zhou X, Liu X, Huang L. Macrophage-Mediated Tumor Cell Phagocytosis: Opportunity for Nanomedicine Intervention. Adv Funct Mater. 2021;31(5):2006220.
Cioffi M, Trabulo S, Hidalgo M, et al. Inhibition of CD47 effectively targets pancreatic cancer stem cells via dual mechanisms. Clin Cancer Res. 2015;21(10):2325–37.
Rao L, Zhao SK, Wen C, et al. Activating macrophage-mediated cancer immunotherapy by genetically edited nanoparticles. Adv Mater. 2020;32(47): e2004853.
Chamseddine AN, Assi T, Mir O, et al. Modulating tumor-associated macrophages to enhance the efficacy of immune checkpoint inhibitors: a TAM-pting approach. Pharmacol Ther. 2021;231:107986.
Takimoto CH, Chao MP, Gibbs C, et al. The Macrophage “Do not eat me” signal, CD47, is a clinically validated cancer immunotherapy target. Ann Oncol. 2019;30(3):486–9.
Bradley CA. CD24—a novel “don’t eat me” signal. Nat Rev Cancer. 2019;19(10):541.
CD24 Is a "don't eat me" signal that promotes tumor immune escape. Cancer Discov. 2019;9(9):1156.
Barkal AA, Brewer RE, Markovic M, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–6.
Li C, Xu X, Wei S, et al. Tumor-associated macrophages: potential therapeutic strategies and future prospects in cancer. J Immunother Cancer. 2021;9(1):e001341.
Barkal AA, Weiskopf K, Kao KS, et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. 2018;19(1):76–84.
Li H, Somiya M, Kuroda S. Enhancing antibody-dependent cellular phagocytosis by Re-education of tumor-associated macrophages with resiquimod-encapsulated liposomes. Biomaterials. 2021;268: 120601.
Klichinsky M, Ruella M, Shestova O, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38(8):947–53.
Santoni M, Massari F, Montironi R, et al. Manipulating macrophage polarization in cancer patients: from nanoparticles to human chimeric antigen receptor macrophages. Biochim Biophys Acta Rev Cancer. 2021;1876(1): 188547.
Abdin SM, Paasch D, Morgan M, et al. CARs and beyond: tailoring macrophage-based cell therapeutics to combat solid malignancies. J Immunother Cancer. 2021;9(8):e002741.
Tie Y, Zheng H, He Z, et al. Targeting folate receptor β positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal Transduct Target Ther. 2020;5(1):6.
Rodriguez-Garcia A, Lynn RC, Poussin M, et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun. 2021;12(1):877.
Ruella M, Klichinsky M, Kenderian SS, et al. Overcoming the Immunosuppressive tumor microenvironment of hodgkin lymphoma using chimeric antigen receptor T cells. Cancer Discov. 2017;7(10):1154–67.
Morrissey MA, Williamson AP, Steinbach AM, et al. Chimeric antigen receptors that trigger phagocytosis. Elife. 2018;7:e36688.
Braster R, O’Toole T, van Egmond M. Myeloid cells as effector cells for monoclonal antibody therapy of cancer. Methods. 2014;65(1):28–37.
Zhang W, Liu L, Su H, et al. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br J Cancer. 2019;121(10):837–45.
Niu Z, Chen G, Chang W, et al. Chimeric antigen receptor-modified macrophages trigger systemic anti-tumour immunity. J Pathol. 2021;253(3):247–57.
Zhang L, Tian L, Dai X, et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J Hematol Oncol. 2020;13(1):153.
Liu Q, Li A, Tian Y, et al. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016;31:61–71.
Faget J, Peters S, Quantin X, et al. Neutrophils in the era of immune checkpoint blockade. J Immunother Cancer. 2021;9(7):e002242.
D’Alterio C, Barbieri A, Portella L, et al. Inhibition of stromal CXCR4 impairs development of lung metastases. Cancer Immunol Immunother. 2012;61(10):1713–20.
Casanova-Acebes M, Nicolás-Ávila JA, Li JL, et al. Neutrophils instruct homeostatic and pathological states in naive tissues. J Exp Med. 2018;215(11):2778–95.
Yang J, Kumar A, Vilgelm AE, et al. Loss of CXCR4 in myeloid cells enhances antitumor immunity and reduces melanoma growth through NK cell and FASL mechanisms. Cancer Immunol Res. 2018;6(10):1186–98.
Devi S, Wang Y, Chew WK, et al. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med. 2013;210(11):2321–36.
Bockorny B, Semenisty V, Macarulla T, et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat Med. 2020;26(6):878–85.
Wu L, Awaji M, Saxena S, et al. IL-17-cxc chemokine receptor 2 axis facilitates breast cancer progression by up-regulating neutrophil recruitment. Am J Pathol. 2020;190(1):222–33.
Coffelt SB, Kersten K, Doornebal CW, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345–8.
Jungnickel C, Schmidt LH, Bittigkoffer L, et al. IL-17C mediates the recruitment of tumor-associated neutrophils and lung tumor growth. Oncogene. 2017;36(29):4182–90.
Gaffen SL, Jain R, Garg AV, et al. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14(9):585–600.
Jin C, Lagoudas GK, Zhao C, et al. Commensal microbiota promote lung cancer development via γδ T Cells. Cell. 2019;176(5):998-1013.e16.
Ocana A, Nieto-Jiménez C, Pandiella A, et al. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. 2017;16(1):137.
Corrales L, Ajona D, Rafail S, et al. Anaphylatoxin C5a creates a favorable microenvironment for lung cancer progression. J Immunol. 2012;189(9):4674–83.
Denk S, Taylor RP, Wiegner R, et al. Complement C5a-induced changes in neutrophil morphology during inflammation. Scand J Immunol. 2017;86(3):143–55.
Raccosta L, Fontana R, Maggioni D, et al. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J Exp Med. 2013;210(9):1711–28.
van Egmond M, Bakema JE. Neutrophils as effector cells for antibody-based immunotherapy of cancer. Semin Cancer Biol. 2013;23(3):190–9.
Brandsma AM, Bondza S, Evers M, et al. Potent Fc receptor signaling by IgA leads to superior killing of cancer cells by neutrophils compared to IgG. Front Immunol. 2019;10:704.
Treffers LW, Ten Broeke T, Rösner T, et al. IgA-mediated killing of tumor cells by neutrophils is enhanced by CD47-SIRPα checkpoint inhibition. Cancer Immunol Res. 2020;8(1):120–30.
Matlung HL, Babes L, Zhao XW, et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 2018;23(13):3946-59.e6.
Ring NG, Herndler-Brandstetter D, Weiskopf K, et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci USA. 2017;114(49):E10578–85.
Mantovani A, Marchesi F, Jaillon S, et al. Tumor-associated myeloid cells: diversity and therapeutic targeting. Cell Mol Immunol. 2021;18(3):566–78.
Cheng Y, Li H, Deng Y, et al. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9(4):422.
Xu W, Dong J, Zheng Y, et al. Immune-checkpoint protein VISTA regulates antitumor immunity by controlling myeloid cell-mediated inflammation and immunosuppression. Cancer Immunol Res. 2019;7(9):1497–510.
Wang L, Rubinstein R, Lines JL, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208(3):577–92.
Yajuk O, Baron M, Toker S, et al. The PD-L1/PD-1 axis blocks neutrophil cytotoxicity in cancer. Cells. 2021;10(6):1510.
Wang TT, Zhao YL, Peng LS, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. 2017;66(11):1900–11.
Baudhuin J, Migraine J, Faivre V, et al. Exocytosis acts as a modulator of the ILT4-mediated inhibition of neutrophil functions. Proc Natl Acad Sci USA. 2013;110(44):17957–62.
Wang J, Shiratori I, Uehori J, et al. Neutrophil infiltration during inflammation is regulated by PILRα via modulation of integrin activation. Nat Immunol. 2013;14(1):34–40.
Feng M, Jiang W, Kim BYS, et al. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. 2019;19(10):568–86.
Jenmalm MC, Cherwinski H, Bowman EP, et al. Regulation of myeloid cell function through the CD200 receptor. J Immunol. 2006;176(1):191–9.
Massara M, Bonavita O, Savino B, et al. ACKR2 in hematopoietic precursors as a checkpoint of neutrophil release and anti-metastatic activity. Nat Commun. 2018;9(1):676.
Wang SJ, Khullar K, Kim S, et al. Effect of cyclo-oxygenase inhibitor use during checkpoint blockade immunotherapy in patients with metastatic melanoma and non-small cell lung cancer. J Immunother Cancer. 2020;8(2):e000889.
Yan J, Smyth MJ, Teng MWL. Interleukin (IL)-12 and IL-23 and their conflicting roles in cancer. Cold Spring Harb Perspect Biol. 2018;10(7):a028530.
Tait Wojno ED, Hunter CA, Stumhofer JS. The immunobiology of the interleukin-12 family: room for discovery. Immunity. 2019;50(4):851–70.
Wang P, Li X, Wang J, et al. Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat Commun. 2017;8(1):1395.
Agliardi G, Liuzzi AR, Hotblack A, et al. Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat Commun. 2021;12(1):444.
Zhang L, Davies JS, Serna C, et al. Enhanced efficacy and limited systemic cytokine exposure with membrane-anchored interleukin-12 T-cell therapy in murine tumor models. J Immunother Cancer. 2020;8(1):e000210.
Eser P, Jänne PA. TGFβ pathway inhibition in the treatment of non-small cell lung cancer. Pharmacol Ther. 2018;184:112–30.
Ciardiello D, Elez E, Tabernero J, et al. Clinical development of therapies targeting TGFβ: current knowledge and future perspectives. Ann Oncol. 2020;31(10):1336–49.
Holmgaard RB, Schaer DA, Li Y, et al. Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer. 2018;6(1):47.
Lan Y, Zhang D, Xu C, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med. 2018;10(424):eaan5488.
Lind H, Gameiro SR, Jochems C, et al. Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances. J Immunother Cancer. 2020;8(1):e000433.
Mahiddine K, Blaisdell A, Ma S, et al. Relief of tumor hypoxia unleashes the tumoricidal potential of neutrophils. J Clin Invest. 2020;130(1):389–403.
Shrestha S, Noh JM, Kim SY, et al. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonist attenuate tumor growth via polarization of neutrophils toward an antitumor phenotype. Oncoimmunology. 2016;5(1): e1067744.
Pylaeva E, Harati MD, Spyra I, et al. NAMPT signaling is critical for the proangiogenic activity of tumor-associated neutrophils. Int J Cancer. 2019;144(1):136–49.
Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–18.
Ferreira LMR, Muller YD, Bluestone JA, et al. Next-generation regulatory T cell therapy. Nat Rev Drug Discov. 2019;18(10):749–69.
Binnewies M, Mujal AM, Pollack JL, et al. Unleashing type-2 dendritic cells to drive protective antitumor CD4(+) T cell immunity. Cell. 2019;177(3):556-71.e16.
Saleh R, Elkord E. FoxP3(+) T regulatory cells in cancer: prognostic biomarkers and therapeutic targets. Cancer Lett. 2020;490:174–85.
Solomon I, Amann M, Goubier A, et al. CD25-T(reg)-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity. Nat Cancer. 2020;1(12):1153–66.
Arce Vargas F, Furness AJS, Solomon I, et al. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity. 2017;46(4):577–86.
Fecci PE, Sweeney AE, Grossi PM, et al. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res. 2006;12(14 Pt 1):4294–305.
Jacobs JF, Punt CJ, Lesterhuis WJ, et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin Cancer Res. 2010;16(20):5067–78.
Oweida A, Hararah MK, Phan A, et al. Resistance to radiotherapy and PD-L1 blockade is mediated by TIM-3 upregulation and regulatory T-cell infiltration. Clin Cancer Res. 2018;24(21):5368–80.
Ji D, Song C, Li Y, et al. Combination of radiotherapy and suppression of Tregs enhances abscopal antitumor effect and inhibits metastasis in rectal cancer. J Immunother Cancer. 2020;8(2):e000826.
Dees S, Ganesan R, Singh S, et al. Regulatory T cell targeting in cancer: emerging strategies in immunotherapy. Eur J Immunol. 2021;51(2):280–91.
Kurose K, Ohue Y, Sato E, et al. Increase in activated Treg in TIL in lung cancer and in vitro depletion of Treg by ADCC using an antihuman CCR4 mAb (KM2760). J Thorac Oncol. 2015;10(1):74–83.
Maeda S, Murakami K, Inoue A, et al. CCR4 blockade depletes regulatory T cells and prolongs survival in a canine model of bladder cancer. Cancer Immunol Res. 2019;7(7):1175–87.
Marshall LA, Marubayashi S, Jorapur A, et al. Tumors establish resistance to immunotherapy by regulating T(reg) recruitment via CCR4. J Immunother Cancer. 2020;8(2):e000764.
Sugiyama D, Nishikawa H, Maeda Y, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci USA. 2013;110(44):17945–50.
Chang AL, Miska J, Wainwright DA, et al. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 2016;76(19):5671–82.
Doi T, Muro K, Ishii H, et al. A Phase I study of the anti-CC chemokine receptor 4 antibody, mogamulizumab, in combination with nivolumab in patients with advanced or metastatic solid tumors. Clin Cancer Res. 2019;25(22):6614–22.
Kurose K, Ohue Y, Wada H, et al. Phase Ia study of FoxP3+ CD4 Treg depletion by infusion of a humanized anti-CCR4 antibody, KW-0761. Cancer Patients Clin Cancer Res. 2015;21(19):4327–36.
Abu-Eid R, Samara RN, Ozbun L, et al. Selective inhibition of regulatory T cells by targeting the PI3K–Akt pathway. Cancer Immunol Res. 2014;2(11):1080–9.
Ali K, Soond DR, Pineiro R, et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510(7505):407–11.
Ahmad S, Abu-Eid R, Shrimali R, et al. Differential PI3Kδ signaling in CD4(+) T-cell subsets enables selective targeting of T regulatory cells to enhance cancer immunotherapy. Cancer Res. 2017;77(8):1892–904.
Qin A, Wen Z, Zhou Y, et al. MicroRNA-126 regulates the induction and function of CD4(+) Foxp3(+) regulatory T cells through PI3K/AKT pathway. J Cell Mol Med. 2013;17(2):252–64.
Shayan G, Srivastava R, Li J, et al. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K–Akt pathway in head and neck cancer. Oncoimmunology. 2017;6(1):e1261779.
Wang X, Wong K, Ouyang W, et al. Targeting IL-10 family cytokines for the treatment of human diseases. Cold Spring Harb Perspect Biol. 2019;11(2):a028548.
Stewart CA, Metheny H, Iida N, et al. Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. J Clin Invest. 2013;123(11):4859–74.
Sawant DV, Yano H, Chikina M, et al. Adaptive plasticity of IL-10(+) and IL-35(+) T(reg) cells cooperatively promotes tumor T cell exhaustion. Nat Immunol. 2019;20(6):724–35.
Wang S, Gao X, Shen G, et al. Interleukin-10 deficiency impairs regulatory T cell-derived neuropilin-1 functions and promotes Th1 and Th17 immunity. Sci Rep. 2016;6:24249.
Chan IH, Wu V, Bilardello M, et al. PEG-rIL-10 treatment decreases FoxP3(+) tregs despite upregulation of intratumoral IDO. Oncoimmunology. 2016;5(7): e1197458.
Naing A, Papadopoulos KP, Autio KA, et al. Safety, antitumor activity, and immune activation of pegylated recombinant human interleukin-10 (AM0010) in patients with advanced solid tumors. J Clin Oncol. 2016;34(29):3562–9.
Naing A, Infante JR, Papadopoulos KP, et al. PEGylated IL-10 (Pegilodecakin) induces systemic immune activation, CD8(+) T cell invigoration and polyclonal T cell expansion in cancer patients. Cancer Cell. 2018;34(5):775-91.e3.
Turnis ME, Sawant DV, Szymczak-Workman AL, et al. Interleukin-35 limits anti-tumor immunity. Immunity. 2016;44(2):316–29.
Han KL, Thomas SV, Koontz SM, et al. Adenosine A2A receptor agonist-mediated increase in donor-derived regulatory T cells suppresses development of graft-versus-host disease. J Immunol. 2013;190(1):458–68.
Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257–65.
Allard B, Longhi MS, Robson SC, et al. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev. 2017;276(1):121–44.
Leone RD, Emens LA. Targeting adenosine for cancer immunotherapy. J Immunother Cancer. 2018;6(1):57.
Ohta A, Kini R, Ohta A, et al. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol. 2012;3:190.
Bao R, Shui X, Hou J, et al. Adenosine and the adenosine A2A receptor agonist, CGS21680, upregulate CD39 and CD73 expression through E2F–1 and CREB in regulatory T cells isolated from septic mice. Int J Mol Med. 2016;38(3):969–75.
Vijayan D, Young A, Teng MWL, et al. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17(12):765.
Young A, Ngiow SF, Madore J, et al. Targeting adenosine in BRAF-mutant melanoma reduces tumor growth and metastasis. Cancer Res. 2017;77(17):4684–96.
Hay CM, Sult E, Huang Q, et al. Targeting CD73 in the tumor microenvironment with MEDI9447. Oncoimmunology. 2016;5(8): e1208875.
Li XY, Moesta AK, Xiao C, et al. Targeting CD39 in cancer reveals an extracellular ATP- and inflammasome-driven tumor immunity. Cancer Discov. 2019;9(12):1754–73.
Masoumi E, Jafarzadeh L, Mirzaei HR, et al. Genetic and pharmacological targeting of A2a receptor improves function of anti-mesothelin CAR T cells. J Exp Clin Cancer Res. 2020;39(1):49.
Beavis PA, Henderson MA, Giuffrida L, et al. Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy. J Clin Invest. 2017;127(3):929–41.
Kim HR, Park HJ, Son J, et al. Tumor microenvironment dictates regulatory T cell phenotype: upregulated immune checkpoints reinforce suppressive function. J Immunother Cancer. 2019;7(1):339.
Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–71.
Moreno Ayala MA, Li Z, DuPage M. Treg programming and therapeutic reprogramming in cancer. Immunology. 2019;157(3):198–209.
Lucca LE, Dominguez-Villar M. Modulation of regulatory T cell function and stability by co-inhibitory receptors. Nat Rev Immunol. 2020;20(11):680–93.
Zhang A, Ren Z, Tseng KF, et al. Dual targeting of CTLA-4 and CD47 on T(reg) cells promotes immunity against solid tumors. Sci Transl Med. 2021;13(605).
Tekguc M, Wing JB, Osaki M, et al. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc Natl Acad Sci USA. 2021;118(30):e2023739118.
Munn DH, Sharma MD, Johnson TS. Treg destabilization and reprogramming: implications for cancer immunotherapy. Cancer Res. 2018;78(18):5191–9.
Marangoni F, Zhakyp A, Corsini M, et al. Expansion of tumor-associated Treg cells upon disruption of a CTLA-4-dependent feedback loop. Cell. 2021;184(15):3998-4015.e19.
Lee JC, Mehdizadeh S, Smith J, et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol. 2020;5(52):eaba0759.
Zappasodi R, Serganova I, Cohen IJ, et al. CTLA-4 blockade drives loss of T(reg) stability in glycolysis-low tumours. Nature. 2021;591(7851):652–8.
Shevach EM, Stephens GL. The GITR–GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat Rev Immunol. 2006;6(8):613–8.
Amoozgar Z, Kloepper J, Ren J, et al. Targeting Treg cells with GITR activation alleviates resistance to immunotherapy in murine glioblastomas. Nat Commun. 2021;12(1):2582.
Yang R, Sun L, Li CF, et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun. 2021;12(1):832.
Mahne AE, Mauze S, Joyce-Shaikh B, et al. Dual roles for regulatory T-cell depletion and costimulatory signaling in agonistic GITR targeting for tumor immunotherapy. Cancer Res. 2017;77(5):1108–18.
Zappasodi R, Sirard C, Li Y, et al. Rational design of anti-GITR-based combination immunotherapy. Nat Med. 2019;25(5):759–66.
Fabian KP, Malamas AS, Padget MR, et al. Therapy of established tumors with rationally designed multiple agents targeting diverse immune-tumor interactions: engage, expand. Enable Cancer Immunol Res. 2021;9(2):239–52.
Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21(4):503–13.
Freeman ZT, Nirschl TR, Hovelson DH, et al. A conserved intratumoral regulatory T cell signature identifies 4-1BB as a pan-cancer target. J Clin Invest. 2020;130(3):1405–16.
Chen S, Lee LF, Fisher TS, et al. Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res. 2015;3(2):149–60.
Joller N, Lozano E, Burkett PR, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40(4):569–81.
Yang ZZ, Kim HJ, Wu H, et al. TIGIT expression is associated with T-cell suppression and exhaustion and predicts clinical outcome and anti-PD-1 response in follicular lymphoma. Clin Cancer Res. 2020;26(19):5217–31.
Wu L, Mao L, Liu JF, et al. Blockade of TIGIT/CD155 signaling reverses T-cell exhaustion and enhances antitumor capability in head and neck squamous cell carcinoma. Cancer Immunol Res. 2019;7(10):1700–13.
Zhang X, Xiao X, Lan P, et al. OX40 costimulation inhibits Foxp3 expression and Treg induction via BATF3-dependent and independent mechanisms. Cell Rep. 2018;24(3):607–18.
Kumar P, Marinelarena A, Raghunathan D, et al. Critical role of OX40 signaling in the TCR-independent phase of human and murine thymic Treg generation. Cell Mol Immunol. 2019;16(2):138–53.
Griffiths J, Hussain K, Smith HL, et al. Domain binding and isotype dictate the activity of anti-human OX40 antibodies. J Immunother Cancer. 2020;8(2):e001557.
Kvarnhammar AM, Veitonmäki N, Hägerbrand K, et al. The CTLA-4 x OX40 bispecific antibody ATOR-1015 induces anti-tumor effects through tumor-directed immune activation. J Immunother Cancer. 2019;7(1):103.
Redmond WL, Linch SN, Kasiewicz MJ. Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Cancer Immunol Res. 2014;2(2):142–53.
Vocanson M, Rozieres A, Hennino A, et al. Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of TH17/TH1 and regulatory T cells. J Allergy Clin Immunol. 2010;126(2):280–9, 89.e1–7.
Martin-Orozco N, Li Y, Wang Y, et al. Melanoma cells express ICOS ligand to promote the activation and expansion of T-regulatory cells. Cancer Res. 2010;70(23):9581–90.
Sim GC, Martin-Orozco N, Jin L, et al. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Invest. 2014;124(1):99–110.
Sim GC, Liu C, Wang E, et al. IL2 variant circumvents ICOS+ regulatory T-cell expansion and promotes NK Cell activation. Cancer Immunol Res. 2016;4(11):983–94.
Zeng H, Yang K, Cloer C, et al. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499(7459):485–90.
Su W, Chapman NM, Wei J, et al. Protein prenylation drives discrete signaling programs for the differentiation and maintenance of effector T(reg) cells. Cell Metab. 2020;32(6):996-1011.e7.
Sainson RCA, Thotakura AK, Kosmac M, et al. An antibody targeting ICOS increases intratumoral cytotoxic to regulatory T-cell ratio and induces tumor regression. Cancer Immunol Res. 2020;8(12):1568–82.
Bol KF, Schreibelt G, Gerritsen WR, et al. Dendritic cell-based immunotherapy: state of the art and beyond. Clin Cancer Res. 2016;22(8):1897–906.
Scheid E, Major P, Bergeron A, et al. Tn-MUC1 DC vaccination of rhesus macaques and a phase I/II trial in patients with nonmetastatic castrate-resistant prostate cancer. Cancer Immunol Res. 2016;4(10):881–92.
Baek S, Kim YM, Kim SB, et al. Therapeutic DC vaccination with IL-2 as a consolidation therapy for ovarian cancer patients: a phase I/II trial. Cell Mol Immunol. 2015;12(1):87–95.
Boudewijns S, Bloemendal M, de Haas N, et al. Autologous monocyte-derived DC vaccination combined with cisplatin in stage III and IV melanoma patients: a prospective, randomized phase 2 trial. Cancer Immunol Immunother. 2020;69(3):477–88.
Rodríguez-Ruiz ME, Perez-Gracia JL, Rodríguez I, et al. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann Oncol. 2018;29(5):1312–9.
Santos PM, Adamik J, Howes TR, et al. Impact of checkpoint blockade on cancer vaccine-activated CD8+ T cell responses. J Exp Med. 2020;217(7):e20191369.
Lövgren T, Wolodarski M, Wickström S, et al. Complete and long-lasting clinical responses in immune checkpoint inhibitor-resistant, metastasized melanoma treated with adoptive T cell transfer combined with DC vaccination. Oncoimmunology. 2020;9(1):1792058.
Lau SP, van Montfoort N, Kinderman P, et al. Dendritic cell vaccination and CD40-agonist combination therapy licenses T cell-dependent antitumor immunity in a pancreatic carcinoma murine model. J Immunother Cancer. 2020;8(2):e000772.
Cifuentes-Rius A, Desai A, Yuen D, et al. Inducing immune tolerance with dendritic cell-targeting nanomedicines. Nat Nanotechnol. 2021;16(1):37–46.
Katakowski JA, Mukherjee G, Wilner SE, et al. Delivery of siRNAs to dendritic cells using DEC205-targeted lipid nanoparticles to inhibit immune responses. Mol Ther. 2016;24(1):146–55.
Zhang H, Zhang J, Li Q, et al. Site-specific MOF-based immunotherapeutic nanoplatforms via synergistic tumor cells-targeted treatment and dendritic cells-targeted immunomodulation. Biomaterials. 2020;245: 119983.
Stead SO, McInnes SJP, Kireta S, et al. Manipulating human dendritic cell phenotype and function with targeted porous silicon nanoparticles. Biomaterials. 2018;155:92–102.
Grover A, Sanseviero E, Timosenko E, et al. Myeloid-derived suppressor cells: a propitious road to clinic. Cancer Discov. 2021;11(11):2693–706.
Acknowledgements
We appreciate the BioRender.com which give us materials about making figures.
Funding
This work was supported by the National Science Fund for Excellent Young Scholars National Science Fund for Excellent Young Scholars (No. 32122052), National Natural Science Foundation Regional Innovation and Development (No. U19A2003).
Author information
Authors and Affiliations
Contributions
XW offered main direction of this manuscript. YW offered significant guidance. YT and FT drafted the manuscript, illustrated the figures, and made tables for the manuscript. YT and FT contributed equally to this work. All author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tie, Y., Tang, F., Wei, Yq. et al. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol Oncol 15, 61 (2022). https://doi.org/10.1186/s13045-022-01282-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-022-01282-8