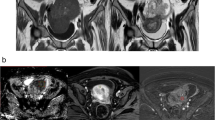
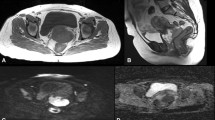
Abstract
Purpose
The purpose of the study was to assess the diagnostic performance of qualitative and quantitative diffusion-weighted imaging (DWI) in differentiating benign from malignant ovarian and uterine masses.
Materials and methods
Institutional review board approval was obtained for this HIPAA-compliant retrospective study, with waiver of informed consent. DWI MRIs of 222 women acquired over 1.5 years were evaluated. Reference standard was pathology or follow-up imaging. For qualitative assessment, two radiologists independently reviewed DWI and apparent diffusion coefficient (ADC) images for diffusion restriction. Differences were resolved by consensus. For quantitative assessment, a single reader measured ADC values. Readers were blinded to the reference standard.
Results
222 lesions, 121 ovarian (99 benign and 22 malignant) and 101 uterine (54 benign and 47 malignant), were included. Final diagnosis was established with pathology in 129 (58%) or with imaging follow-up in 93 (42%). Mean (range) follow-up interval was 27 (13–48) months. Qualitative assessment yielded sensitivity (ratio, 95% CI), specificity, PPV and NPV of 100% (22/22, 85–100), 68% (68/99, 58–76), 41% (22/54, 27–54), and 100% (68/68, 94–100) for ovarian and 94% (44/47, 83–98), 91% (49/54, 80–96), 90% (44/49, 78–95) and 94% (49/52, 84–98) for uterine malignancies. ADC (mean ± SD) between benign ovarian [(1.11 ± 0.76) × 10−3 mm2/s] vs. malignant [(0.71 ± 0.26) × 10−3 mm2/s] lesions was significantly different (p < 0.001). ADC cutoff value of 1.55 × 10−3 mm2/s for ovarian lesions resulted in 99.9% confidence for the absence of malignancy. ADC (mean ± SD) of benign uterine [(0.64 ± 0.38) × 10−3 mm2/s] vs. malignant [(0.68 ± 0.19) × 10−3 mm2/s] lesions was not significantly different (P < 0.54).
Conclusion
Quantitative and qualitative DWI assessment can be used to confidently characterize a subset of ovarian lesions as benign. With uterine lesions, although DWI is useful in differentiating benign from malignant lesions, the technique does not allow for definitive quantitative characterization.





Similar content being viewed by others
References
Kinkel K, Lu Y, Mehdizade A, Pelte MF, Hricak H (2005) Indeterminate ovarian mass at US: incremental value of second imaging test for characterization—meta-analysis and Bayesian analysis. Radiology 236(1):85–94
Anthoulakis C, Nikoloudis N (2014) Pelvic MRI as the “gold standard” in the subsequent evaluation of ultrasound-indeterminate adnexal lesions: a systematic review. Gynecol Oncol 132(3):661–668
Iyer VR, Lee SI (2010) MRI, CT, and PET/CT for ovarian cancer detection and adnexal lesion characterization. AJR Am J Roentgenol 194(2):311–321
Cornfeld D, Israel G, Martel M, et al. (2010) MRI appearance of mesenchymal tumors of the uterus. Eur J Radiol 74(1):241–249
Goto A, Takeuchi S, Sugimura K, Maruo T (2002) Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer 12(4):354–361
Harry VN (2010) Novel imaging techniques as response biomarkers in cervical cancer. Gynecol Oncol 116(2):253–261
Kilickesmez O, Cimilli T, Inci E, et al. (2009) Diffusion-weighted MRI of urinary bladder and prostate cancers. Diagn Interv Radiol 15(2):104–110
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 188(6):1622–1635
McVeigh PZ, Syed AM, Milosevic M, Fyles A, Haider MA (2008) Diffusion-weighted MRI in cervical cancer. Eur Radiol 18(5):1058–1064
Beddy P, Moyle P, Kataoka M, et al. (2012) Evaluation of depth of myometrial invasion and overall staging in endometrial cancer: comparison of diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology 262(2):530–537
Donati OF, Chong D, Nanz D, et al. (2014) Diffusion-weighted MR imaging of upper abdominal organs: field strength and intervendor variability of apparent diffusion coefficients. Radiology 270(2):454–463
Kim S, Jain M, Harris AB, et al. (2009) T1 hyperintense renal lesions: characterization with diffusion-weighted MR imaging versus contrast-enhanced MR imaging. Radiology 251(3):796–807
Namimoto T, Awai K, Nakaura T, et al. (2009) Role of diffusion-weighted imaging in the diagnosis of gynecological diseases. Eur Radiol 19(3):745–760
Fujii S, Kakite S, Nishihara K, et al. (2008) Diagnostic accuracy of diffusion-weighted imaging in differentiating benign from malignant ovarian lesions. J Magn Reson Imaging 28(5):1149–1156
Thomassin-Naggara I, Darai E, Cuenod CA, et al. (2009) Contribution of diffusion-weighted MR imaging for predicting benignity of complex adnexal masses. Eur Radiol 19(6):1544–1552
Li W, Chu C, Cui Y, Zhang P, Zhu M (2012) Diffusion-weighted MRI: a useful technique to discriminate benign versus malignant ovarian surface epithelial tumors with solid and cystic components. Abdom Imaging 37(5):897–903
Tamai K, Koyama T, Saga T, et al. (2008) The utility of diffusion-weighted MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol 18(4):723–730
Landis JR, Koch GG (1977) An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 33(2):363–374
Song JS, Hwang SB, Chung GH, Jin GY (2016) Intra-individual, inter-vendor comparison of diffusion-weighted mr imaging of upper abdominal organs at 3.0 Tesla with an emphasis on the value of normalization with the spleen. Korean J Radiol 17(2):209–217
Inada Y, Matsuki M, Nakai G, et al. (2009) Body diffusion-weighted MR imaging of uterine endometrial cancer: is it helpful in the detection of cancer in nonenhanced MR imaging? Eur J Radiol 70(1):122–127
Naganawa S, Sato C, Kumada H, et al. (2005) Apparent diffusion coefficient in cervical cancer of the uterus: comparison with the normal uterine cervix. Eur Radiol 15(1):71–78
Tamai K, Koyama T, Saga T, et al. (2007) Diffusion-weighted MR imaging of uterine endometrial cancer. J Magn Reson Imaging 26(3):682–687
Weinreb JC, Barentsz JO, Choyke PL, et al. (2016) PI-RADS Prostate Imaging-Reporting and Data System: 2015, Version 2. Eur Urol 69(1):16–40
Mitchell DG, Bruix J, Sherman M, Sirlin CB (2015) LI-RADS (Liver Imaging Reporting and Data System): Summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 61(3):1056–1065
Thomassin-Naggara I, Aubert E, Rockall A, et al. (2013) Adnexal masses: development and preliminary validation of an MR imaging scoring system. Radiology 267(2):432–443
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None reported by any author.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The Institutional Review Board at our institution approved this single-center retrospective study and the need for informed consent was waived.
Rights and permissions
About this article
Cite this article
Davarpanah, A.H., Kambadakone, A., Holalkere, N.S. et al. Diffusion MRI of uterine and ovarian masses: identifying the benign lesions. Abdom Radiol 41, 2466–2475 (2016). https://doi.org/10.1007/s00261-016-0909-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0909-2