Abstract
Objectives
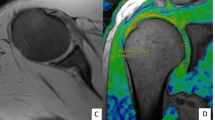
We compare the T1 and T2 relaxation times and magnetization transfer ratios (MTRs) of normal subjects and patients with osteoarthritis (OA) to evaluate the ability of these techniques to aid in the early diagnosis and treatment of OA.
Materials and methods
The knee joints in 11 normal volunteers and 40 patients with OA were prospectively evaluated using T1 relaxation times as measured using delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), T2 relaxation times (multiple spin-echo sequence, T2 mapping), and MTRs. The OA patients were further categorized into mild, moderate, and severe OA.
Results
The mean T1 relaxation times of the four groups (normal, mild OA, moderate OA, and severe OA) were: 487.3 ± 27.7, 458.0 ± 55.9, 405.9 ± 57.3, and 357.9 ± 36.7 respectively (p <0.001). The mean T2 relaxation times of the four groups were: 37.8 ± 3.3, 44.0 ± 8.5, 50.9 ± 9.5, and 57.4 ± 4.8 respectively (p < 0.001). T1 relaxation time decreased and T2 relaxation time increased with worsening degeneration of patellar cartilage. The result of the covariance analysis showed that the covariate age had a significant influence on T2 relaxation time (p < 0.001). No significant differences between the normal and OA groups using MTR were noted.
Conclusion
T1 and T2 relaxation times are relatively sensitive to early degenerative changes in the patellar cartilage, whereas the MTR may have some limitations with regard to early detection of OA. In addition, The T1 and T2 relaxation times negatively correlate with each other, which is a novel finding.



Similar content being viewed by others
References
Grushko G, Schneiderman R, Maroudas A. Some biochemical and biophysical parameters for the study of the pathogenesis of osteoarthritis: a comparison between the processes of ageing and degeneration in human hip cartilage. Connect Tiss Res. 1989;19:149–76.
Lohmander LS. Articular cartilage and osteoarthrosis. The role of molecular markers to monitor breakdown, repair and disease. J Anat. 1994;184:477–92.
Buckwalter JA, Martin J. Degenerative joint disease. Clin Symp. 1995;47:1–32.
Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36(5):665–73.
Bashir A, Gray ML, Hartke J, et al. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41(5):857–65.
Nieminen MT, Rieppo J, Toyras J, et al. T2 relaxation time reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. 2001;46(3):487–93.
Smith HE, Mosher TJ, Dardzinski BJ, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging. 2001;14(1):50–5.
Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40.
Kellgren JH, Lawrence JS. Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;16(4):494–501.
Dardzinski BJ, Mosher TJ, Li S, et al. Spatial variation of T2 in human articular cartilage. Radiology. 1997;205(2):546–50.
Xia Y. Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol. 2000;35(10):602–21.
Lusse S, Claassen H, Gehrke T, et al. Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging. 2000;18(4):423–30.
Mosher TJ, Chen Q, Smith MB. 1H magnetic resonance spectroscopy of nanomelic chicken cartilage: effect of aggrecan depletion on cartilage T2. Osteoarthritis Cartilage. 2003;11(10):709–15.
Fragonas E, Mlynarik V, Jellus V, et al. Correlation between biochemical composition and magnetic resonance appearance of articular cartilage. Osteoarthritis Cartilage. 1998;6(1):24–32.
Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2—preliminary findings at 3 T. Radiology. 2000;214(1):259–66.
Watson PJ, Carpenter TA, Hall LD, et al. Cartilage swelling and loss in a spontaneous model of osteoarthritis visualized by magnetic resonance imaging. Osteoarthritis Cartilage. 1996;4(3):197–207.
Gahunia HK, Lemaire C, Babyn PS, et al. Osteoarthritis in rhesus macaque knee joint: quantitative magnetic resonance imaging tissue characterization of articular cartilage. J Rheumatol. 1995;22(9):1747–56.
Dunn TC, Lu Y, Jin H, et al. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232(2):592–8.
Regatte RR, Akella SV, Lonner JH, et al. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23(4):547–53.
Bashir A, Gray ML, Burstein RD, et al. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)2-enhanced MR imaging. Radiology. 1997;205(2):551–8.
Mlynárik V, Trattnig S, Huber M, et al. The role of relaxation times in monitoring proteoglycan depletion in articular cartilage. J Magn Reson Imaging. 1999;10(4):497–502.
Burstein D, Velyvis J, Scott KT, et al. Protocol issues for delayed Gd(DTPA)2-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45(1):36–41.
Van Breuseghem I. Ultrastructural MR imaging techniques of the knee articular cartilage: problems for routine clinical application. Eur Radiol. 2004;14(2):184–92.
Lammentausta E, Kiviranta P, Nissi MJ, et al. T2 relaxation time and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) of human patellar cartilage at 1.5 T and 9.4 T: relationships with tissue mechanical properties. J Orthop Res. 2006;24(3):366–74.
Tiderius CJ, Olsson LE, de Verdier H, et al. Gd-DTPA2-enhanced MRI of femoral knee cartilage: a dose-response study in healthy volunteers. Magn Reson Med. 2001;46(6):1067–71.
Nissi MJ, Töyräs J, Laasanen MS, et al. Proteoglycan and collagen sensitive MRI evaluation of normal and degenerated articular cartilage. J Orthop Res. 2004;22(3):557–64.
Palmieri F, De Keyzer F, Maes F, et al. Magnetization transfer analysis of cartilage repair tissue: a preliminary study. Skeletal Radiol. 2006;35(12):903–8.
Stanisz GJ, Odrobina EE, Pun J, et al. T1, T2 relaxation time and magnetization transfer in tissue at 3 T. Magn Reson Med. 2005;54(3):507–12.
Martirosian P, Boss A, Deimling M. Systematic variation of off-resonance prepulses for clinical magnetization transfer contrast imaging at 0.2, 1.5, and 3.0 tesla. Invest Radiol. 2008;43(1):16–26.
Regatte RR, Akella SV, Reddy R. Depth-dependent proton magnetization transfer in articular cartilage. J Magn Reson Imaging. 2005;22(2):318–23.
Petersen EF, Fishbein KW, Laouar L, et al. Ex vivo magnetic resonance microscopy of an osteochondral transfer. J Magn Reson Imaging. 2003;17:603–8.
Laurent D, Wasvary J, Yin Jin, et al. Quantitative and qualitative assessment of articular cartilage in the goat knee with magnetization transfer imaging. Magn Reson Imaging. 2001;19(10):1279–86.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yao, W., Qu, N., Lu, Z. et al. The application of T1 and T2 relaxation time and magnetization transfer ratios to the early diagnosis of patellar cartilage osteoarthritis. Skeletal Radiol 38, 1055–1062 (2009). https://doi.org/10.1007/s00256-009-0769-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-009-0769-8