Abstract
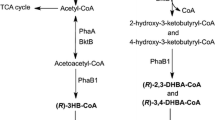
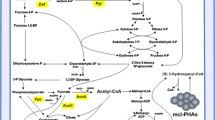
This work describes the generation of novel PHAs (named PHACOS) with a new monomer composition containing thioester groups in the side chain, which confers new properties and made them suitable for chemical modifications after their biosynthesis. We have analyzed the PHACOS production abilities of the wild-type strain Pseudomonas putida KT2442 vs. its derived strain P. putida KT42FadB, mutated in the fadB gene from the central metabolic β-oxidation pathway involved in the synthesis of medium-chain-length PHA (mcl-PHA). Different fermentation strategies based on one- or two-stage cultures have been tested resulting in PHACOS with different monomer composition. Using decanoic acid as inducer of the growth and polymer synthesis and 6-acetylthiohexanoic acid as PHA precursor in a two-stage strategy, the maximum yield was obtained by culturing the strain KT42FadB. Nuclear magnetic resonance and gas chromatography coupled to mass spectrometry showed that polymers obtained from the wild-type and KT42FadB strains, included 6-acetylthio-3-hydroxyhexanoic acid (OH-6ATH) and the shorter derivative 4-acetylthio-3-hydroxybutanoic acid (OH-4ATB) in their composition, although in different ratios. While the polymer obtained from KT42FadB strain contained mainly OH-6ATH monomer units, mcl-PHA produced by the wild-type strain contained OH-6ATH and OH-4ATB. Furthermore, polyesters showed differences in the OH-alkyl derivates moiety. The strain KT42FadB overproduced PHACOS when compared to the production rate of the control strain in one- and two-stage cultures. Thermal properties obtained by differential scanning calorimetry indicated that both polymers have different glass transition temperatures related to their composition.






Similar content being viewed by others
References
Bear M-M, Leboucher-Durand M-A, Langlois V, Lenz RW, Goodwin S, Guérin P (1997) Bacterial poly-3-hydroxyalkenoates with epoxy groups in the side chains. React Funct Polym 34(1):65–77
Bertani G (2004) Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J Bacteriol 186(3):595–600
Chen GQ (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38(8):2434–2446
Chen GQ, Wu Q (2005a) The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 26(33):6565–6578
Chen GQ, Wu Q (2005b) Microbial production and applications of chiral hydroxyalkanoates. Appl Microbiol Biotechnol 67(5):592–599
de Eugenio LI, Escapa IF, Morales V, Dinjaski N, Galán B, García JL, Prieto MA (2010a) The turnover of medium-chain-length polyhydroxyalkanoates in Pseudomonas putida KT2442 and the fundamental role of PhaZ depolymerase for the metabolic balance. Environ Microbiol 12(1):207–221
de Eugenio LI, Galán B, Escapa IF, Maestro B, Sanz JM, García JL, Prieto MA (2010b) The PhaD regulator controls the simultaneous expression of the pha genes involved in polyhydroxyalkanoate metabolism and turnover in Pseudomonas putida KT2442. Environ Microbiol 12(6):1591–1603
Doi Y, Abe C (1990) Biosynthesis and characterization of a new bacterial copolyester of 3-hydroxyalkanoates and 3-hydroxy-ω-chloroalkanoates. Macromolecules 23(15):3705–3707
Durner R, Zinn M, Witholt B, Egli T (2001) Accumulation of poly[(R)-3-hydroxyalkanoates] in Pseudomonas oleovorans during growth in batch and chemostat culture with different carbon sources. Biotechnol Bioeng 72(3):278–288
Ewering C, Lütke-Eversloh T, Luftmann H, Steinbüchel A (2002) Identification of novel sulfur-containing bacterial polyesters: biosynthesis of poly(3-hydroxy-S-propyl-ω-thioalkanoates) containing thioether linkages in the side chains. Microbiology 148(Pt 5):1397–1406
Fritzsche K, Lenz RW, Fuller RC (1990) Production of unsaturated polyesters by Pseudomonas oleovorans. Int J Biol Macromol 12(2):85–91
García B, Olivera ER, Miñambres B, Fernández-Valverde M, Cañedo LM, Prieto MA, García JL, Martínez M, Luengo JM (1999) Novel biodegradable aromatic plastics from a bacterial source. Genetic and biochemical studies on a route of the phenylacetyl-CoA catabolon. J Biol Chem 274(41):29228–29241
Grant SG, Jessee J, Bloom FR, Hanahan D (1990) Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci USA 87(12):4645–4649
Hartmann R, Hany R, Pletscher E, Ritter A, Witholt B, Zinn M (2006) Tailor-made olefinic medium-chain-length poly[(R)-3-hydroxyalkanoates] by Pseudomonas putida GPo1: batch versus chemostat production. Biotechnol Bioeng 93(4):737–746
Hartmann R, Hany R, Witholt B, Zinn M (2010) Simultaneous biosynthesis of two copolymers in Pseudomonas putida GPo1 using a two-stage continuous culture system. Biomacromolecules 11(6):1488–1493
Hazer B (2010) Amphiphilic poly(3-hydroxy alkanoate)s: potential candidates for medical applications. Int J Polym Sci 2010:8. doi:10.1155/2010/423460
Hazer B, Steinbüchel A (2007) Increased diversification of polyhydroxyalkanoates by modification reactions for industrial and medical applications. Appl Microbiol Biotechnol 74(1):1–12
Hazer B, Lenz RW, Fuller RC (1994) Biosynthesis of methyl-branched poly(β-hydroxyalkanoate)s by Pseudomonas oleovorans. Macromolecules 27(1):45–49
Herrero M, de Lorenzo V, Timmis KN (1990) Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in Gram-negative bacteria. J Bacteriol 172(11):6557–6567
Huijberts GN, Eggink G, de Waard P, Huisman GW, Witholt B (1992) Pseudomonas putida KT2442 cultivated on glucose accumulates poly(3-hydroxyalkanoates) consisting of saturated and unsaturated monomers. Appl Environ Microbiol 58(2):536–544
Huisman GW, Wonink E, Meima R, Kazemier B, Terpstra P, Witholt B (1991) Metabolism of poly(3-hydroxyalkanoates) (PHAs) by Pseudomonas oleovorans. Identification and sequences of genes and function of the encoded proteins in the synthesis and degradation of PHA. J Biol Chem 266(4):2191–2198
Hunter P (2010) Can bacteria save the planet? EMBO Rep 11(4):266–269
Kim O, Gross RA, Hammar WJ, Newmark RA (1996) Microbial synthesis of poly(β-hydroxyalkanoates) containing fluorinated side-chain substituents. Macromolecules 29(13):4572–4581
Lageveen RG, Huisman GW, Preusting H, Ketelaar P, Eggink G, Witholt B (1988) Formation of polyesters by Pseudomonas oleovorans: effect of substrates on formation and composition of poly-(R)-3-hydroxyalkanoates and poly-(R)-3-hydroxyalkenoates. Appl Environ Microbiol 54(12):2924–2932
Lenz RW, Marchessault RH (2005) Bacterial polyesters: biosynthesis, biodegradable plastics and biotechnology. Biomacromolecules 6(1):1–8
Lenz RW, Kim YB, Fuller RC (1992) Production of unusual bacterial polyesters by Pseudomonas oleovorans through cometabolism. FEMS Microbiol Lett 103(2–4):207–214
Lütke-Eversloh T, Steinbüchel A (2004) Microbial polythioesters. Macromol Biosci 4(3):166–174
Lütke-Eversloh T, Fischer A, Remminghorst U, Kawada J, Marchessault RH, Bögershausen A, Kalwei M, Eckert H, Reichelt R, Liu S-J, Steinbüchel A (2002) Biosynthesis of novel thermoplastic polythioesters by engineered Escherichia coli. Nat Mater 1(4):236–240
Moldes C, García P, García JL, Prieto MA (2004) In vivo immobilization of fusion proteins on bioplastics by the novel tag BioF. Appl Environ Microbiol 70(6):3205–3212
Nelson KE, Weinel C, Paulsen IT, Dodson RJ, Hilbert H, Martins dos Santos VA, Fouts DE, Gill SR, Pop M, Holmes M, Brinkac L, Beanan M, DeBoy RT, Daugherty S, Kolonay J, Madupu R, Nelson W, White O, Peterson J, Khouri H, Hance I, Chris Lee P, Holtzapple E, Scanlan D, Tran K, Moazzez A, Utterback T, Rizzo M, Lee K, Kosack D, Moestl D, Wedler H, Lauber J, Stjepandic D, Hoheisel J, Straetz M, Heim S, Kiewitz C, Eisen JA, Timmis KN, Düsterhöft A, Tümmler B, Fraser CM (2002) Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ Microbiol 4(12):799–808
Nogales J, Palsson BO, Thiele I (2008) A genome-scale metabolic reconstruction of Pseudomonas putida KT2440: iJN746 as a cell factory. BMC Syst Biol 2:79
Olivera ER, Carnicero D, García B, Miñambres B, Moreno MA, Cañedo L, Dirusso CC, Naharro G, Luengo JM (2001a) Two different pathways are involved in the β-oxidation of n-alkanoic and n-phenylalkanoic acids in Pseudomonas putida U: genetic studies and biotechnological applications. Mol Microbiol 39(4):863–874
Olivera ER, Carnicero D, Jodra R, Miñambres B, García B, Abraham GA, Gallardo A, Román JS, García JL, Naharro G, Luengo JM (2001b) Genetically engineered Pseudomonas: a factory of new bioplastics with broad applications. Environ Microbiol 3(10):612–618
Ouyang SP, Liu Q, Fang L, Chen GQ (2007) Construction of pha-operon-defined knockout mutants of Pseudomonas putida KT2442 and their applications in poly(hydroxyalkanoate) production. Macromol Biosci 7(2):227–233
Park WH, Lenz RW, Goodwin S (1998) Epoxidation of bacterial polyesters with unsaturated side chains. I. Production and epoxidation of polyesters from 10-undecenoic acid. Macromolecules 31(5):1480–1486
Prieto MA, de Eugenio LI, Galán B, Luengo JM, Witholt B (2007) Synthesis and degradation of polyhydroxyalkanoates. In: Ramos JL, Filloux A (eds) Pseudomonas: a model system in biology. Pseudomonas, vol V. Springer, Berlin
Puchalka J, Oberhardt MA, Godinho M, Bielecka A, Regenhardt D, Timmis KN, Papin JA, Martins dos Santos VA (2008) Genome-scale reconstruction and analysis of the Pseudomonas putida KT2440 metabolic network facilitates applications in biotechnology. PLoS Comput Biol 4(10):e1000210
Rehm BH (2010) Bacterial polymers: biosynthesis, modifications and applications. Nat Rev Microbiol 8(8):578–592
Ren Q, Ruth K, Thöny-Meyer L, Zinn M (2010) Enatiomerically pure hydroxycarboxylic acids: current approaches and future perspectives. Appl Microbiol Biotechnol 87(1):41–52
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sandoval A, Arias-Barrau E, Bermejo F, Cañedo L, Naharro G, Olivera ER, Luengo JM (2005) Production of 3-hydroxy-n-phenylalkanoic acids by a genetically engineered strain of Pseudomonas putida. Appl Microbiol Biotechnol 67(1):97–105
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145(1):69–73
Scholz C (2010) Perspectives to produce positively or negatively charged polyhydroxyalkanoic acids. Appl Microbiol Biotechnol 88(4):829–837
Scholz C, Fuller RC, Lenz RW (1994a) Growth and polymer incorporation of Pseudomonas oleovorans on alkyl esters of heptanoic acid. Macromolecules 27(10):2886–2889
Scholz C, Wolk S, Lenz RW, Fuller RC (1994b) Growth and polyester production by Pseudomonas oleovorans on branched octanoic acid substrates. Macromolecules 27(22):6358–6362
Sendil D, Gürsel I, Wise DL, Hasirci V (1999) Antibiotic release from biodegradable PHBV microparticles. J Control Release 59(2):207–217
Steinbüchel A, Valentin HE (1995) Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol Lett 128(3):219–228
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 25(10):1503–1555
Takagi Y, Hashii M, Maehara A, Yamane T (1999) Biosynthesis of polyhydroxyalkanoate with a thiophenoxy side group obtained from Pseudomonas putida. Macromolecules 32(25):8315–8318
Tyo KE, Zhou H, Stephanopoulos GN (2006) High-throughput screen for poly-3-hydroxybutyrate in Escherichia coli and Synechocystis sp. strain PCC6803. Appl Environ Microbiol 72(5):3412–3417
Valappil SP, Misra SK, Boccaccini AR, Roy I (2006) Biomedical applications of polyhydroxyalkanoates: an overview of animal testing and in vivo responses. Expert Rev Med Devices 3(6):853–868
Witholt B, Kessler B (1999) Perspectives of medium chain length poly(hydroxyalkanoates), a versatile set of bacterial bioplastics. Curr Opin Biotechnol 10(3):279–285
Zinn M, Witholt B, Egli T (2001) Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv Drug Deliv Rev 53(1):5–21
Acknowledgements
We thank Dr. E. Díaz for helpful discussions. We are indebted to Marta Tortajada from Biopolis S.L. for sending us the standard mcl-PHA. The technical works of A. Valencia are greatly appreciated. This work was supported by grants from the Ministry of Science and Innovation (BIO2007-67304, BIO2010-21049, CSD2007-00005) and by European Union Grants (GEN 2006-27750-C5-3-E and NMP2-CT-2007-026515). Isabel F. Escapa is a recipient of CSIC-I3P predoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Escapa, I.F., Morales, V., Martino, V.P. et al. Disruption of β-oxidation pathway in Pseudomonas putida KT2442 to produce new functionalized PHAs with thioester groups. Appl Microbiol Biotechnol 89, 1583–1598 (2011). https://doi.org/10.1007/s00253-011-3099-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3099-4