Abstract
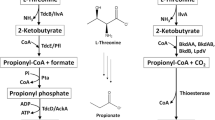
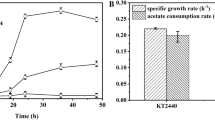
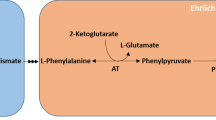
Overexpression of the gene encoding the poly-3-hydroxy-n-phenylalkanoate (PHPhA) depolymerase (phaZ) in Pseudomonas putida U avoids the accumulation of these polymers as storage granules. In this recombinant strain, the 3-OH-acyl-CoA derivatives released from the different aliphatic or aromatic poly-3-hydroxyalkanoates (PHAs) are catabolized through the β-oxidation pathway and transformed into general metabolites (acetyl-CoA, succinyl-CoA, phenylacetyl-CoA) or into non-metabolizable end-products (cinnamoyl-CoA). Taking into account the biochemical, pharmaceutical and industrial interest of some PHA catabolites (i.e., 3-OH-PhAs), we designed a genetically engineered strain of P. putida U (P. putida U ΔfadBA-phaZ) that efficiently bioconverts (more than 80%) different n-phenylalkanoic acids into their 3-hydroxyderivatives and excretes these compounds into the culture broth.





Similar content being viewed by others
References
Abraham GA, Gallardo A, San Román J, Olivera ER, Jodrá R, García B, Miñambres B, García JL, Luengo JM (2001) Microbial síntesis of poly(β-hydroxyalkanoates) bearing phenylgroups from Pseudomonas putida: chemical structure and characterization. Biomacromolecules 2:562–567
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Burke T, Chandrasekhar B, Knight M (1999) Analogs of viscosin and uses thereof. Peptide Technologies Corp., Washington, D.C.
Cardoso CR, Brito FCF de, Silva KCM da, Miranda ALP de, Fraga CAM, Barreiro EJ (2002) Design, síntesis and pharmacological evaluation of novel pyrazolo[3,4-b]thieno[2,3-d]pyridine acid derivatives: a new class of anti-inflammatory and anti-platelet agents. Bioorg Med Chem Lett 12:9–12
De Boer J, Backer JH (1967) Diazomethane. Org Synth Coll 4:250–253
De Roo G, Kellerhals MB, Ren Q, Witholt B, Kessler B (2002) Production of chiral R-3-hydroxyalkanoic acids and R-3-hydroxyalkanoic acid methylesters via hydrolytic degradation of polyhydroxyalkanoate synthesized by pseudomonads. Biotechnol Bioeng 77:717–722
Dieuleveux V, Pyl D van der, Chataud J, Gueguen M (1998) Purification and characterization of anti-Listeria compounds produced by Geotrichum candidum. Appl Environ Microbiol 64:800–803
Fiedler S, Steinbüchel A, Rehm BHA (2002) The role of the fatty acid β-oxidation multienzyme complex from Pseudomonas oleovorans in polyhydroxyalkanoates biosynthesis: molecular characterization of the fadBA operon from P. oleovorans and of the enoyl-CoA hydratase genes PhaJ from P. oleovorans and Pseudomonas putida. Arch Microbiol 178:149–160
Floriano B, Ruiz Barba JL, Jiménez-Díaz R (1998) Purification and genetic characterization of enterocin I from Enterococcus faecium 6T1a, a novel antilisterial plasmid-encoded bacteriocin which does not belong to the pediocin family of bacteriocins. Appl Environ Microbiol 64:4883–4890
Fritzsche K, Lentz RW, Fuller RC (1990) An unusual bacterial polyester with a phenyl pendant group. Makromol Chem 191:1957–1965
Fukui T, Shiomi N, Doi Y (1998) Expression and characterization of (R)-specific enoyl coenzyme A hydratase involved in polyhydroxyalkanoates biosynthesis by Aeromonas caviae. J Bacteriol 180:667–673
García B (2004) Biosíntesis of polihidroxialcanoatos en Pseudomonas putida U: caracterización genética y bioquímica del sistema responsable de su acúmulo y movilización. PhD thesis, Universidad de León, León
García B, Olivera ER, Miñambres B, Fernández-Valverde M, Cañedo LM, Prieto MA, García JL, Martínez M, Luengo JM (1999) Novel biodegradable aromatic plastics from a bacterial source. J Biol Chem 274:29228–29241
Herrero M, Lorenzo V de, Timmis KN (1990) Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol 172:6557–6567
Jendrossek D, Handrick R (2002) Microbial degradation of polyhydroxyalkanoates. Annu Rev Microbiol 56:403–432
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176
Lageveen RG, Huisman GW, Preusting H, Ketelaar P, Eggink G, Witholt B (1988) Formation of polyesters by Pseudomonas oleovorans. Effect of substrates on formation and composition of poly-(R)-3-hydroxyalkanoates and poly-(R)-3-hydroxyalkenoates. Appl Environ Microbiol 54:2924–2932
Luengo JM, García JL, Olivera ER (2001) The phenylacetyl-CoA catabolón: a complex catabolic unit with broad biotechnological applications. Mol Microbiol 39:1434–1442
Luengo JM, García B, Sandoval A, Naharro G, Olivera E (2003) Bioplastics from microorganisms. Curr Opin Microbiol 6:251–260
Madison LL, Huisman GW (1999) Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev 63:21–53
Martínez-Blanco H, Reglero A, Rodríguez-Aparicio LB, Luengo JM (1990) Purification and biochemical characterization of phenylacetyl-CoA ligase from Pseudomonas putida U. A specific enzyme for the catabolism of phenylacetic acid. J Biol Chem 265:7084–7090
Miñambres B, Olivera ER, García B, Naharro G, Luengo JM (2000) From a short sequence to the complete genome. Biochem Biophys Res Commun 272:477–479
Moore JA, Reed DE (1973) Diazomethane. Org Synth Coll 5:351–355
Olivera ER, Miñambres B, García B, Muñiz C, Moreno MA, Ferrández A, Díaz E, García JL, Luengo JM (1998) Molecular characterization of the phenylacetic acid catabolic pathway in Pseudomonas putida U: the phenylacetyl-CoA catabolón. Proc Natl Acad Sci USA 95:6419–6424
Olivera ER, Carnicero D, Jodrá R, Miñambres B, García B, Abraham GA, Gallardo A, San Román J, García JL, Luengo JM (2001a) Genetically engineered Pseudomonas: a factory of new bioplastics with broad applications. Environ Microbiol 3:612–618
Olivera ER, Carnicero B, García B, Miñambres B, Moreno MA, Cañedo L, DiRusso CC, Naharro G, Luengo JM (2001b) Two different pathways are involved in the β-oxidation of n-alkanoic and n-phenylalkanoic acids in Pseudomonas putida U: genetic studies and biotechnological applications. Mol Microbiol 39:863–874
Peypoux F, Bonmatin JM, Wallach J (1999) Recent trends in the biochemistry of surfactin. Appl Microbiol Biotechnol 51:553–563
Redemann CE, Rice FO, Roberts R, Ward HP (1967) Diazomethane. Org Synth Coll 3:244–248
Rehm BHA (2003) Polyesters synthases: natural catalysts for plastics. Biochem J 376:15–33
Rehm BHA, Krüger N, Steinbüchel A (1998) A new metabolic link between fatty acid de novo synthesis and polyhydroxyalkanoic acid synthesis. J Biol Chem 273:24044–24051
Steinbüchel A (2001) Perspectives for biotechnological production and utilization of biopolymers: metabolic engineering of polyhydroxyalkanoates biosynthesis pathway as a successful example. Macromol Biosci 1:1–24
Sudesh K, Gan Z, Matsumoto K, Doi Y (2002) Direct observation of polyhydroxyalkanoate chains by atomic force microscopy. Ultramicroscopy 91:157–164
Tsuge T, Fukui T, Matsusaki H, Taguchi S, Kobayashi G, Ishizaki A, Doi Y (1999) Molecular cloning of two (R)-specific enoyl-CoA hydratases genes from Pseudomonas aeruginosa and their use for polyhydroxyalkanoates synthesis. FEMS Microbiol Lett 184:193–198
Tsuge T, Taguchi K, Taguchi S, Doi Y (2003) Molecular characterization and properties of (R)-specific enoyl-CoA hydratases from Pseudomonas aeruginosa: metabolic tools for synthesis of polyhydroxyalkanoates via fatty acid β-oxidation. Biol Macromol 31:195–205
Vaysse L, Ly A, Moulin G, Dubreucq E (2002) Chain-length selectivity of various lipases during hydrolysis, esterification and alcoholysis in biphasic aqueous medium. Enzyme Microb Technol 31:648–655
Witholt B, Kessler B (1999) Perspectives of medium chain length poly(hydroxyalkanoates), a versatile set of bacterial bioplastics. Curr Opin Biotechnol 10:279–285
Acknowledgements
This investigation was supported by the Comisión Interministerial de Ciencia y Tecnología (CICYT), Madrid, Spain (grant BIO2003-05309-C04-01). A.S. and E.A. are recipients of fellowships from the Universidad de León and CICYT, respectively.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
NMR data for 3-hydroxy-5-phenylpentanoic acid
1H NMR (CDCl3): δ=1.74 (m; 2H, H-4) 2.45 (m; 2H, H-2), 2.74 (m; 2H, H-5), 4.05 (s, broad; 1H, H-3), 7.17–7.27 (m; 5H, aromatic H). 13C NMR (CDCl3): δ=32.09 (C-5), 37.56 (C-4), 41.27 (C-2), 67.38(C-3), 125.60 (C-4′), 128.31(C-2′, C-6′), 128.21 (C-3′, C-5′), 141.55 (C-1′), 175.97 (C-1).
NMR data for 3-hydroxy-6-phenylhexanoic acid
1H NMR (CDCl3): δ=1.55 (m; 2H, H-4), 1.68–1.81 (m; 2H, H-5), 2.54 (m; 2H, H-2), 2.66 (m; 2H, H-6), 4.08 (s, broad; 1H, H-3), 7.30–7.20 (m; 5H, aromatic H). 13C NMR (CDCl3): δ=27.50 (C-5), 35.86 (C-6), 36.12 (C-4), 41.41 (C-2), 68.20 (C-3), 126.10 (C-4′), 128.67 (C-2′, C-6′), 128.62 (C-3′, C-5′), 142.32 (C-1′), 178.39 (C-1).
NMR data for 3-hydroxy-7-phenylheptanoic acid
1H NMR (CDCl3): δ=1.39–1.49 (m, 2H, H-5), 1.53 (m; 2H, H-4), 1.65 (m, 2H, H-6), 2.47 (m; 2H, H-2), 2.62 (m; 2H, H-7), 4.05 (s, broad; 1H, H-3), 7.17–7.27 (m; 5H, aromatic H). 13C NMR (CDCl3): δ=25.10 (C-5), 31.30 (C-6), 35.79 (C-7), 36.28 (C-4), 41.18 (C-2), 68.04(C-3), 125.66 (C-4′), 128.36 (C-2′, C-6′), 128.26 (C-3′, C-5′), 142.45 (C-1′), 176.04 (C-1).
NMR data for 3-hydroxy-8-phenyloctanoic acid
1H NMR (CDCl3): δ=1.36 (m; 2H, H-6), 1.37–1.47 (m; 2H, H-5), 1.47–1.53 (m; 2H, H-4), 1.63 (m; 2H, H-7), 2.51 (m; 2H, H-2), 2.63 (m; 2H, H-8), 4.03 (s, broad; 1H, H-3), 7.18–7.28 (m; 5H, aromatic H). 13C NMR (CDCl3): δ=25.29 (C-5), 29.07 (C-6), 31.35 (C-7), 35.83 (C-8), 36.30 (C-4), 41.09 (C-2), 68.02 (C-3), 125.63 (C-4′), 128.39 (C-2′, C-6′), 128.25 (C-3′, C-5′), 142.62 (C-1′), 178.06 (C-1).
Rights and permissions
About this article
Cite this article
Sandoval, Á., Arias-Barrau, E., Bermejo, F. et al. Production of 3-hydroxy-n-phenylalkanoic acids by a genetically engineered strain of Pseudomonas putida. Appl Microbiol Biotechnol 67, 97–105 (2005). https://doi.org/10.1007/s00253-004-1752-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1752-x