Abstract
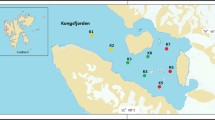
We assessed the diversity and distribution of fungi in 13 water samples collected from four aquatic environments (stream, pond, melting ice water, and estuary) in the Ny-Ålesund Region, Svalbard (High Arctic) using 454 pyrosequencing with fungi-specific primers targeting the internal transcribed spacer (ITS) region of the ribosomal rRNA gene. Aquatic fungal communities in this region showed high diversity, with a total of 43,061 reads belonging to 641 operational taxonomic units (OTUs) being found. Of these OTUs, 200 belonged to Ascomycota, 196 to Chytridiomycota, 120 to Basidiomycota, 13 to Glomeromycota, and 10 to early diverging fungal lineages (traditional Zygomycota), whereas 102 belonged to unknown fungi. The major orders were Helotiales, Eurotiales, and Pleosporales in Ascomycota; Chytridiales and Rhizophydiales in Chytridiomycota; and Leucosporidiales and Sporidiobolales in Basidiomycota. The common fungal genera Penicillium, Rhodotorula, Epicoccum, Glaciozyma, Holtermanniella, Betamyces, and Phoma were identified. Interestingly, the four aquatic environments in this region harbored different aquatic fungal communities. Salinity, conductivity, and temperature were important factors in determining the aquatic fungal diversity and community composition. The results suggest the presence of diverse fungal communities and a considerable number of potentially novel fungal species in Arctic aquatic environments, which can provide reliable data for studying the ecological and evolutionary responses of fungi to climate change in the Arctic ecosystem.




Similar content being viewed by others
References
Overpeck J, Hughen K, Hardy D, Bradley R, Case R et al (1997) Arctic environmental change of the last four centuries. Science 278:1251–1256
Vincent WF (2010) Microbial ecosystem responses to rapid climate change in the Arctic. ISME J 4:1089–1091
Shearer CA, Descals E, Kohlmeyer B, Kohlmeyer J, Marvanova L et al (2007) Fungal biodiversity in aquatic habitats. Biodivers Conserv 16:49–67
Shearer CA, Langsam DM, Longcore JE (2004) Fungi in freshwater habitats. In: Mueller GM, Bills GF, Foster MS (eds) Biodiversity of Fungi: Inventory and Monitoring Methods. Elsevier Academic Press, San Diego, pp 513–532
Wong MKM, Goh TK, Hodgkiss IJ, Hyde KD, Ranghoo VM et al (1998) Role of fungi in freshwater ecosystems. Biodivers Conserv 7:1187–1206
Jones EBG, Pang KL (2012) Tropical aquatic fungi. Biodivers Conserv 21:2403–2423
Mahmoud YAG, Abou-Zeid AM (2002) Zoosporic fungi isolated from four egyptian lakes and the uptake of radioactive waste. Mycobiology 30:76–81
Manoharachary C, Ramarao P (1981) Seasonal variation and distribution of fungi in two freshwater ponds of Andhra Pradesh, India. Proc Indian Acad Sci (Plant Sci) 90:237–243
Jobard M, Rasconi S, Solinhac L, Cauchie HM, Sime-Ngando T (2012) Molecular and morphological diversity of fungi and the associated functions in three European nearby lakes. Environ Microbiol 14:2480–2494
Cai L, Tsui CKM, Zhang K, Hyde KD (2002) Aquatic fungi from Lake Fuxian, Yunnan, China. Fungal Diver 9:57–70
Kagami M, Amano Y, Ishii N (2012) Community structure of planktonic fungi and the impact of parasitic chytrids on phytoplankton in Lake Inba, Japan. Microb Ecol 63:358–368
Chen M, Chen F, Yu Y, Ji J, Kong F (2008) Genetic diversity of eukaryotic microorganisms in Lake Taihu, a large shallow subtropical lake in China. Microb Ecol 56:572–583
Kobayasi Y, Hiratsuka N, Korf RP, Tubaki K, Aoshima K et al (1967) Mycological studies of the Alaskan Arctic. Ann Rep Inst Ferment, Osaka 3:1–138
Sonjak S, Frisvad JC, Gunde-Cimerman N (2006) Penicillium mycobiota in Arctic subglacial ice. Microb Ecol 52:207–216
Butinar L, Spencer-Martins I, Gunde-Cimerman N (2007) Yeasts in high Arctic glaciers: the discovery of a new habitat for eukaryotic microorganisms. Anton Leeuwenhoek Int J G 91:277–289
Gulis V, Baschien C, Marvanová L (2012) Two new Tricladium species from streams in Alaska. Mycologia 104:1510–1516
Ellis-Evans JC (1985) Fungi from maritime Antarctic freshwater environments. Br Antarct Surv Bull 68:37–45
Ellis-Evans JC (1996) Microbial diversity and function in Antarctic freshwater ecosystems. Biodivers Conserv 5:1395–1431
Gonçalves VN, Vaz ABM, Rosa CA, Rosa LH (2012) Diversity and distribution of fungal communities in lakes of Antarctica. FEMS Microbiol Ecol 82:459–471
Monchy S, Sanciu G, Jobard M, Rasconi S, Gerphagnon M et al (2011) Exploring and quantifying fungal diversity in freshwater lake ecosystems using rDNA cloning/sequencing and SSU tag pyrosequencing. Environ Microbiol 13:1433–1453
Duarte S, Barlocher F, Trabulo J, Cassio F, Pascoal C (2015) Stream-dwelling fungal decomposer communities along a gradient of eutrophication unraveled by 454 pyrosequencing. Fungal Diver 70:127–148
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols, a guide to methods and applications. Academic, New York, pp 315–322
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200
Bastian M, Heymann S, Jacomy M (2009) Gephi, an open source software for exploring and manipulating networks. In: Adar E et al (eds) International AAAI Conference on Weblogs and Social Media. AAAI Press, Palo Alto, pp 361–362
Bjorbækmo MFM, Carlsen T, Brysting A, Vrålstad T, Høiland K et al (2010) High diversity of root associated fungi in both alpine and arctic Dryas octopetala. BMC Plant Biol 10:244
Walker JF, Aldrich-Wolfe L, Riffel A, Barbare H, Simpson NB et al (2011) Diverse Helotiales associated with the roots of three species of Arctic Ericaceae provide no evidence for host specificity. New Phytol 191:515–527
Shearer CA, Raja HA, Miller AN, Nelson P, Tanaka K et al (2009) The molecular phylogeny of freshwater Dothideomycetes. Stud Mycol 64:145–153
Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkmann-Kohlmeyer B et al (2009) Molecular systematics of the marine Dothideomycetes. Stud Mycol 64:155–173
Turchetti B, Buzzini P, Goretti M, Branda E, Diolaiuti G et al (2007) Psychrophilic yeasts in glacial environments of Alpine glaciers. FEMS Microbiol Ecol 63:73–83
Wuczkowski M, Passoth V, Turchetti B, Andersson AC, Olstorpe M et al (2011) Description of Holtermanniella gen. nov., including Holtermanniella takashimae sp. nov. and four new combinations, and proposal of the order Holtermanniales to accommodate Tremellomycetous yeasts of the Holtermannia clade. Int J Syst Evol Microbiol 61:680–689
Powell MJ (1993) Looking at mycology with a Janus face. A glimpse at Chytridiomycetes active in the environment. Mycologia 85:1–20
Ibelings BW, De Bruin A, Kagami M, Rijkeboer M, Brehm M et al (2004) Host parasite interactions between freshwater phytoplankton and chytrid fungi (Chytridiomycota). J Phycol 40:437–453
Botnen S, Vik U, Carlsen T, Eidesen PB, Davey ML et al (2014) Low host specificity of root-associated fungi at an Arctic site. Mol Ecol 23:975–985
Day MJ, Gibas CFC, Fujimura KE, Egger KN, Currah RS (2006) Monodictys arctica, a new hyphomycete from the roots of Saxifraga oppositifolia collected in the Canadian High Arctic. Mycotaxon 98:261–272
Geml J, Timling I, Robinson CH, Lennon N, Nusbaum HC et al (2012) An arctic community of symbiotic fungi assembled by long‐distance dispersers: phylogenetic diversity of ectomycorrhizal basidiomycetes in Svalbard based on soil and sporocarp DNA. J Biogeogr 39:74–88
Timling I, Dahlberg A, Walker DA, Gardes M, Charcosset JY et al (2012) Distribution and drivers of ectomycorrhizal fungal communities across the North American Arctic. Ecosphere 3:111
Turchetti B, Hall SRT, Connelll LB, Branda E, Buzzini P et al (2011) Psychrophilic yeasts from Antarctica and European glaciers: description of Glaciozyma gen. nov., Glaciozyma martinii sp. nov. and Glaciozyma watsonii sp. nov. Extremophiles 15:573–586
Zalar P, Gunde-Cimerman N (2014) Cold-adapted yeasts in Arctic habitats. In: Cold-adapted yeasts. Springer Berlin Heidelberg, New York, pp 49–74
Egidi E, De Hoog GS, Isola D, Onofri S, Quaedvliey W et al (2014) Phylogeny and taxonomy of meristematic rock-inhabiting black fungi in the Dothideomycetes based on multi-locus phylogenies. Fungal Diver 65:127–165
Davey ML, Currah RS (2009) Atradidymella muscivora gen. et sp. nov. (Pleosporales) and its anamorph Phoma muscivora sp. nov.: a new pleomorphic pathogen of boreal bryophytes. Am J Bot 96:1281–1288
Rosa LH, Vieira MLA, Santiago IF, Rosa CA (2010) Endophytic fungi community associated with the dicotyledonous plant Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) in Antarctica. FEMS Microbiol Ecol 73:178–189
Baldy V, Chauvet E, Charcosset J, Gessner MO (2002) Microbial dynamics associated with leaves decomposing in the mainstem and floodplain pond of a large river. Aquat Microb Ecol 28:25–36
Gulis V, Suberkropp K (2004) Effects of whole-stream nutrient enrichment on the concentration and abundance of aquatic hyphomycete conidia in transport. Mycologia 96:57–65
Misra JK (1982) Occurrence, distribution and seasonality of aquatic fungi as affected by chemical factors in six alkaline ponds of India. Hydrobiologia 97:185–191
Medeiros AO, Pascoal C, Graça MAS (2009) Diversity and activity of aquatic fungi under low oxygen conditions. Freshwater Biol 54:142–149
Shearer CA (1972) Fungi of the Chesapeake Bay and its tributaries. III. The distribution of wood-inhabiting ascomycetes and fungi imperfecti of the Patuxent River. Am J Bot 59:961–969
Manoharachary C, Ramarao P (1981) Seasonal variation and distribution of fungi in two freshwater ponds of Andhra Pradesh, India. Proceedings: Plant Sciences 90:237–243
Acknowledgments
This research was supported by the National Infrastructure of Microbial Resources (No.NIMR-2015-3), the National Natural Science Foundation of China (NSFC) (Nos. 31170041 and 31300115), the Polar Strategic Research Foundation of China (No. 20120302), and Projects of the Chinese Arctic and Antarctic Administration, State Oceanic Administration (Nos. 2013YR06006 and 2013YR05005). Li-Yan Yu is supported by Xiehe scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zhang, T., Wang, NF., Zhang, YQ. et al. Diversity and Distribution of Aquatic Fungal Communities in the Ny-Ålesund Region, Svalbard (High Arctic). Microb Ecol 71, 543–554 (2016). https://doi.org/10.1007/s00248-015-0689-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-015-0689-1